Pakorn Tony KANCHANAWONG
Associate Professor, Mechanobiology Institute, National University of Singapore
biekp@nus.edu.sg
09-03-08
Level 9 T-Lab
National University of Singapore
5A Engineering Drive 1
Singapore 117411
Laboratory website
Kanchanawong Lab @ NUS
Research Program
Molecular Mechanics of Mechanotransduction Group
Affiliations
Department of Biomedical Engineering, National University of Singapore
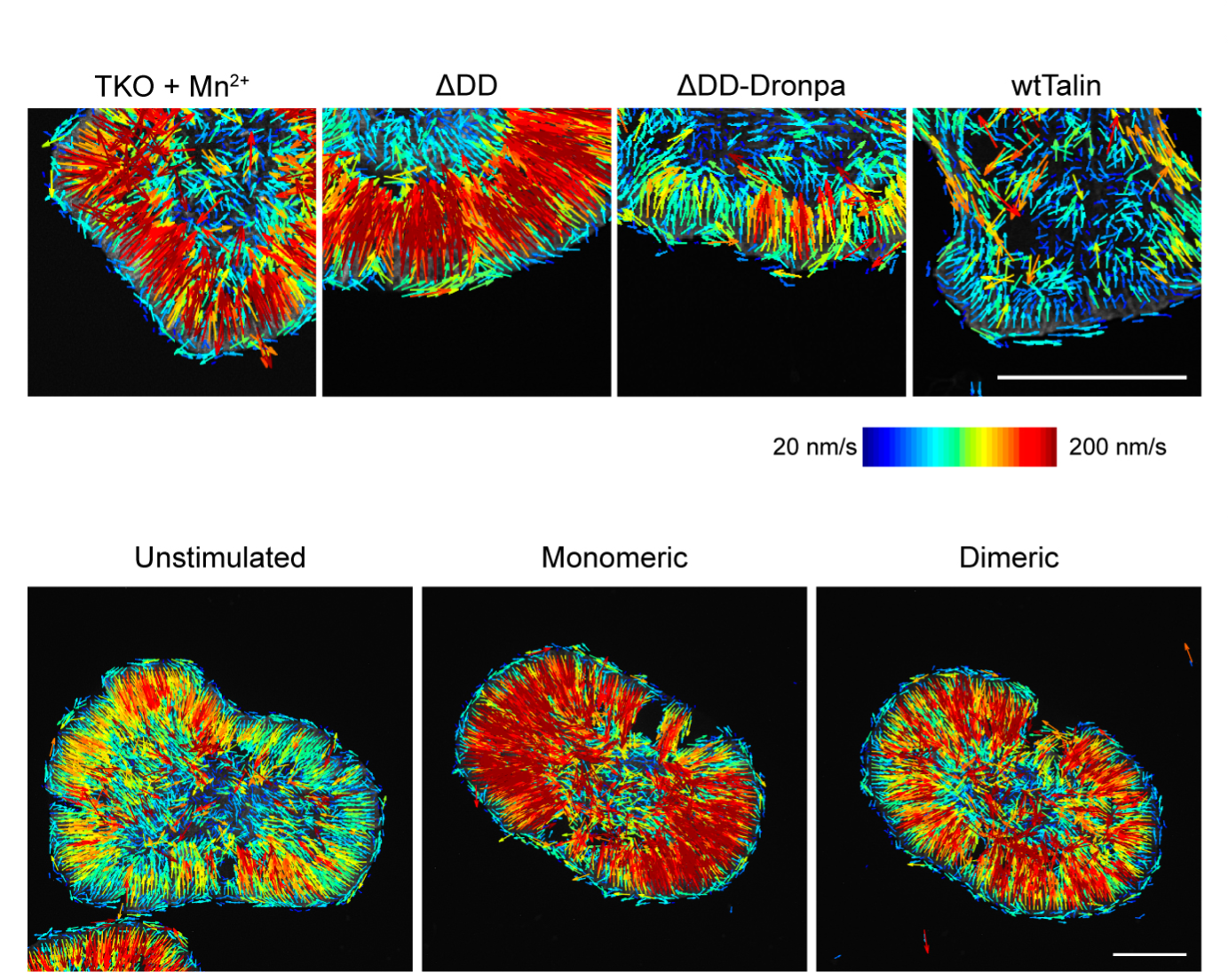
Violet vs. Blue: Controlling Mechanotransduction with a Single-protein Light Switch
In a study published in the Journal of Cell Science, led by Ryosuke Nishimura at the Mechanobiology Institute, NUS, researchers developed an optogenetic tool to precisely manipulate talin’s structure and observe the resulting cellular behavior.
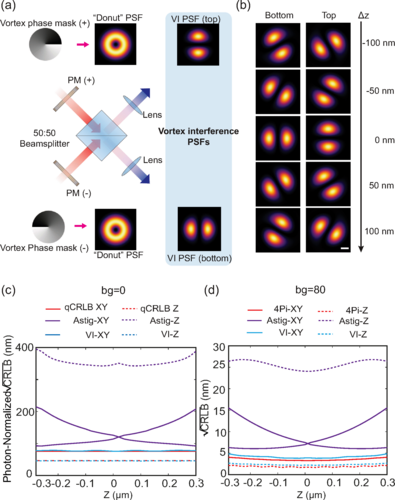
Less is More: Simplified 3D Nanoscopy via Vortex Interference Widens Access
Researchers from the Kanchanawong Lab at the Mechanobiology Institute, NUS makes cutting-edge 3D nanoscopy more accessible to researchers without elaborate optical engineering.
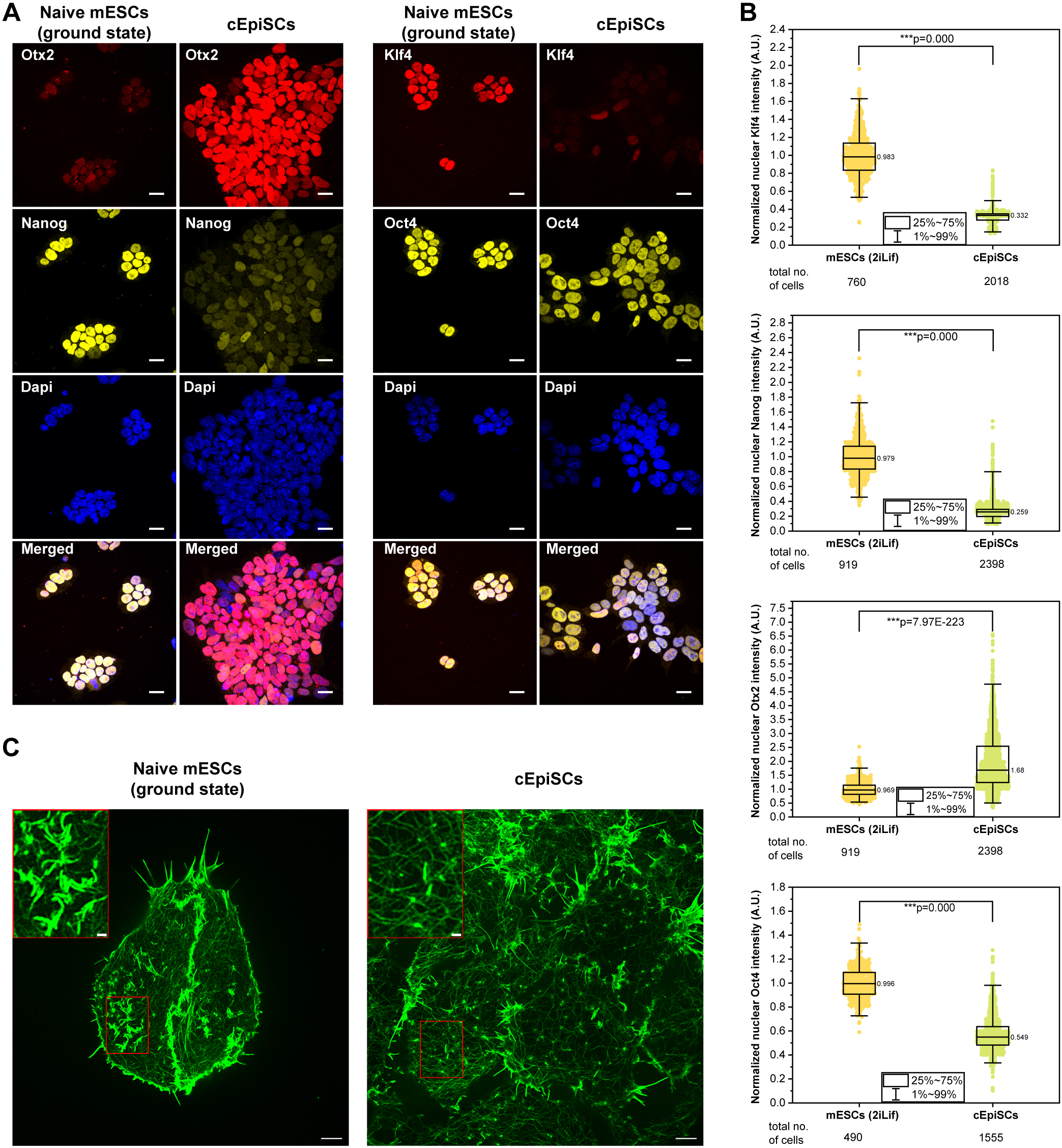
Rac-1 Regulated Cadherin Clusters Mark Naive Stem Cells
Researchers from the Kanchanawong Lab at the Mechanobiology Institute, NUS discover a marker of ground-state pluripotent stem cells and what governs it.
MBI PIs win MOE AcRF Tier 3 grant to study endomembrane aging
A research team led by Mechanobiology Institute (MBI) Principal Investigators Assoc. Prof. Tony Kanchanawong and Prof. Rong Li was recently awarded a Ministry of Education Academic Research Fund (MOE AcRF) Tier 3 grant.
Molecular controllers of stem cell mechanics
Recent study led by MBI graduate student Shumin Xia and Principal Investigator Associate Professor Pakorn Tony Kanchanawong use super resolution imaging techniques to study the nanoscale organization of the cortical actin cytoskeleton in mouse embryonic stem cells and understand the role of molecules such as Arp2/3, formins, and capping protein in coordinating cortical architecture in mESCs.
The guiding hands (and feet)
Focal adhesions in embryonic stem cells
Pakorn Tony Kanchanawong
Principal Investigator
Research Areas
Nanoscale Structure-Function Relationship, Super-resolution Microscopy, Cell-Matrix Interactions, Cell-Cell Interactions, Cellular Biophysics, Mechanobiology of Cell Adhesions and Cell Cortex, Computer Vision, Machine Learning, Bioimaging Technology, Bioimage Informatics.
Research Interests
Cells built complex nanoscale ‘machines’ from basic biomolecular building blocks to perform vital biological functions. These cellular ‘machines’ are at the heart of key processes in mechanobiology, such as cell migration, cell adhesion, and mechanotransduction. Our overarching research goal is to gain a comprehensive insight into the nanoscale structure-function relationship that governs the assembly, organization, dynamics, and functions of these cellular machines. Our approach is highly interdisciplinary, combining advanced imaging technologies with rigorous molecular and cell biology methods.
Super-resolution microscopy
We have pioneered the use of superresolution microscopy to elucidate nanoscale architecture of cellular structures (Kanchanawong et al., Nature, 2010), and have long-standing involvement in the development of ultra-high resolution 3D imaging techniques, iPALM (Shtengel et al., PNAS 2009). Our focus is in advancing the capability of super-resolution microscopy, using several platforms including our own iPALM system and surface-generated structured illumination techniques.
Bioimage informatics
Super-resolution and advanced microscopy techniques generate beautiful, complex, and exquisitely detailed images of cells in large quantity. These images contain vast amount of information but it is still very challenging to quantitatively, rigorously, and comprehensively analyse such datasets. To fully tap the potential of these 21st century imaging techniques, this analysis bottleneck must be tackled. We have several ongoing projects where we seek to leverage computer vision and machine learning approaches to unlock information contained in super-resolution microscopy images (for example: Zhang et al., MBoC 2017).
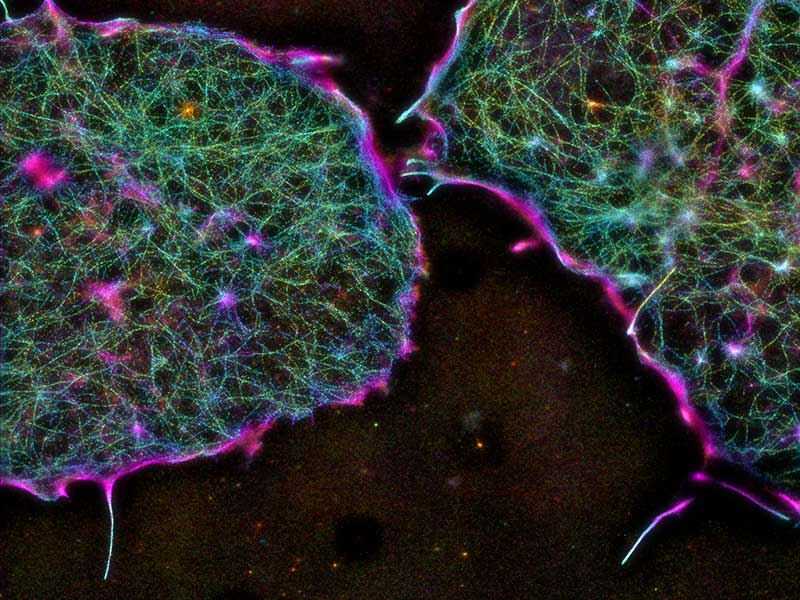
Focal adhesions
Focal adhesions are major cell adhesion structures that mediate cell-extracellular matrix (ECM) adhesions. Focal adhesions play essential roles in mechanotransduction, rigidity sensing, and cell migration. Our focus is in understanding the molecular architecture of focal adhesions (Kanchanawong et al., Nature, 2010) and how they are animated during cellular functions. Our recent work established the roles of the protein Talin as the determinant of focal adhesions architecture (Liu et al., PNAS 2015). Ongoing projects seek to combine nanoscale imaging with molecular engineering approaches to understand the operational principles that control focal adhesions structure and functions.
Cell-cell junctions
In tissues, coherent organization of cells depends on cadherin-mediated cell-cell junctions. We have recently elucidated for the nanoscale architecture of cadherin-based cell adhesions, using superresolution microscopy (Bertocchi et al., Nature Cell Biology, 2017; Wu et al., Developmental Cell, 2015). In our ongoing projects we seek to understand comprehensively the transformation and linkages between nanoscale structures and functions during the formation and maturation of epithelial tissues
Education
PhD (Biophysics) Stanford University
Biography
Tony Kanchanawong received his Bachelor’s degree (A.B. summa cum laude, 2001) from Cornell University where he double-majored in Chemistry and Biological Sciences. At Cornell, he also studied the Molecular Dynamics of cellulase enzymes in the laboratory of Prof. John W. Brady Jr. Going west to Stanford University, he worked on Non-Classical Stark spectroscopy of photosynthetic reaction centers and GFPs with Prof. Steven G. Boxer, supported by the HHMI Predoctoral Fellowship. In 2007, he received his doctorate in Biophysics and became a postdoctoral fellow in the laboratory of Dr. Clare Waterman at NIH, where he closely collaborated with Dr. Harald Hess at HHMI Janelia Research Campus in the development and application of iPALM 3-D superresolution microscopy. In 2011, Tony started his research group at MBI and NUS Department of Biomedical Engineering, as one of the NRF fellowship recipients.
Recent Publications
- Nishimura R, Barnett SFH, Jain K, Huang Z, Goult BT, and Kanchanawong P. Optogenetic control of mechanotransduction based on light-induced homodimerization of talin. J Cell Sci 2025;. [PMID: 41277405]
- Nishimura R, and Kanchanawong P. Nanoscale mechano-adaption of integrin-based cell adhesions: New tools and techniques lead the way. Curr Opin Cell Biol 2025; 94:102509. [PMID: 40188780]
- Wang W, Huang Z, Wang Y, Li H, and Kanchanawong P. Vortex Interference Enables Optimal 3D Interferometric Nanoscopy. Phys Rev Lett 2025; 134(7):073802. [PMID: 40053988]
- Liu S, Meng Y, Lan X, Li R, and Kanchanawong P. Ground-state pluripotent stem cells are characterized by Rac1-dependent Cadherin-enriched F-actin Complexes. J Cell Sci 2025;. [PMID: 39886806]
- Xiao J, Ang JW, Zhong X, Wong DCP, T T, Yow I, Lee CJM, Foo RS, Kanchanawong P, and Low BC. Coordination of Focal Adhesion Nanoarchitecture and Dynamics in Mechanosensing for Cardiomyoblast Differentiation. ACS Appl Mater Interfaces 2025;. [PMID: 39778877]
- Jain K, Kishan K, Minhaj RF, Kanchanawong P, Sheetz MP, and Changede R. Immobile Integrin Signaling Transit and Relay Nodes Organize Mechanosignaling through Force-Dependent Phosphorylation in Focal Adhesions. ACS Nano 2025;. [PMID: 39760672]
- Lin K, Gujar MR, Lin J, Ding WY, Huang J, Gao Y, Tan YS, Teng X, Christine LSL, Kanchanawong P, Toyama Y, and Wang H. Astrocytes control quiescent NSC reactivation via GPCR signaling-mediated F-actin remodeling. Sci Adv 2024; 10(30):eadl4694. [PMID: 39047090]
- Morales-Camilo N, Liu J, Ramírez MJ, Canales-Salgado P, Alegría JJ, Liu X, Ong HT, Barrera NP, Fierro A, Toyama Y, Goult BT, Wang Y, Meng Y, Nishimura R, Fong-Ngern K, Low CSL, Kanchanawong P, Yan J, Ravasio A, and Bertocchi C. Alternative molecular mechanisms for force transmission at adherens junctions via β-catenin-vinculin interaction. Nat Commun 2024; 15(1):5608. [PMID: 38969637]
- Aureille J, Prabhu SS, Barnett SF, Farrugia AJ, Arnal I, Lafanechère L, Low BC, Kanchanawong P, Mogilner A, and Bershadsky AD. Focal adhesions are controlled by microtubules through local contractility regulation. EMBO J 2024;. [PMID: 38769437]
- Jain K, Minhaj RF, Kanchanawong P, Sheetz MP, and Changede R. Nano-clusters of ligand-activated integrins organize immobile, signalling active, nano-clusters of phosphorylated FAK required for mechanosignaling in focal adhesions. bioRxiv 2024;. [PMID: 38464288]
Lab Members
ZHAO Jiayu
PhD Student, Class of August 2025, Kanchanawong Group
Pratchaya Rukthanapitak
Research Associate, Kanchanawong Group
Ryosuke Nishimura
Research Fellow, Kanchanawong Group
Li Hangfeng
PhD Student, Class of August 2022, Kanchanawong Group
Xu Mengqing
PhD Student, Class of August 2021, Kanchanawong Group
Huang Zengxin
PhD Student, Class of August 2021, Kanchanawong Group
Kuo Xuan
PhD Student, Class of August 2021, Kanchanawong Group, Zhao Group
Wang Yilin
Research Fellow, Kanchanawong Group
Meng Yue
Research Assistant, Class of January 2021, Kanchanawong Group
Kedsarin Fong Ngern
Research Fellow, Kanchanawong Group
Zhong Xueying
PhD Student, Class of August 2018, Kanchanawong Group
Wang Wei
Research Fellow, Kanchanawong Group