Research @ MBI
Understanding the molecular basis for mechanotransduction
In cells and tissues, the integration and propagation of mechanical signals is facilitated by the activity of molecular machines; small groups of proteins that detect and respond to mechanical stimuli by transferring physical forces to other cellular components, or facilitating their conversion to biochemical signals.
The information obtained during this process, which is known as mechanosensing, helps in cellular decision making.This is particularly important during development, when stem cells are differentiating to become specific cell types, and during wound healing or tissue repair.
At MBI, we are exploring mechano-transduction though four major research programs: molecular, cellular, tissue, and through technological innovations.
Cells can measure the stiffness of the surface on which they are growing and they can detect and respond to tension from neighboring cells within a tissue. Understanding how individual cells and proteins contribute to the mechanotransduction of physical force, is a major focus in the research conducted at the MBI. Dissecting the nanoscale architecture of various molecular machines involves the manipulation of specific cellular components, and at times, single proteins or specific protein domains. We can then monitor any subsequent effects.
Crucial to these efforts is the ability to control and modify the physical parameters of the cellular microenvironment. This means growing cells on substrates of a specific stiffness, pattern or shape. The effect of any molecular manipulation must then be monitored by quantifying the forces generated by cells or individual proteins, or visualizing the effects using super-resolution microscopy techniques.
Molecular Mechanisms of Mechanobiology
At MBI, we investigate how groups of proteins come together to form modular functional units that are capable of mediating diverse cellular functions by sensing and relaying mechanical signals between various components of the cell. More
Cell-Matrix / Cell-Cell Mechanotransduction
MBI is working to understand how a cell’s behavior within a tissue is guided by its communication with neighboring cells and the extracellular matrix through the formation of protein-based adhesion complexes. More
Mechanotransduction in Tissue Development
At the MBI, we apply biophysical principles to study the highly-coordinated orchestration of cellular events in a tissue, and understand its relevance during the development of an embryo as well as during tissue repair in adult organisms. More
Technology Innovation for Mechanobiology
The state-of-the-art technology at MBI has expanded our understanding of cell mechanics, enabling us to manipulate the physical properties of the cellular microenvironment as well as to precisely quantify cellular response to mechanical signals. More
Recent Featured Research
Microtubules and Cell Movement: A Closer Look at Focal Adhesion Disassembly
Researchers from the Bershadsky Lab at MBI utilized optogenetics to unlock the role of microtubules in regulating focal adhesion disassembly, an important step in cell migration. http://www.mbi.nus.edu.sg/featured-research/microtubules-and-cell-movement-a-closer-look-at-focal-adhesion-disassembly
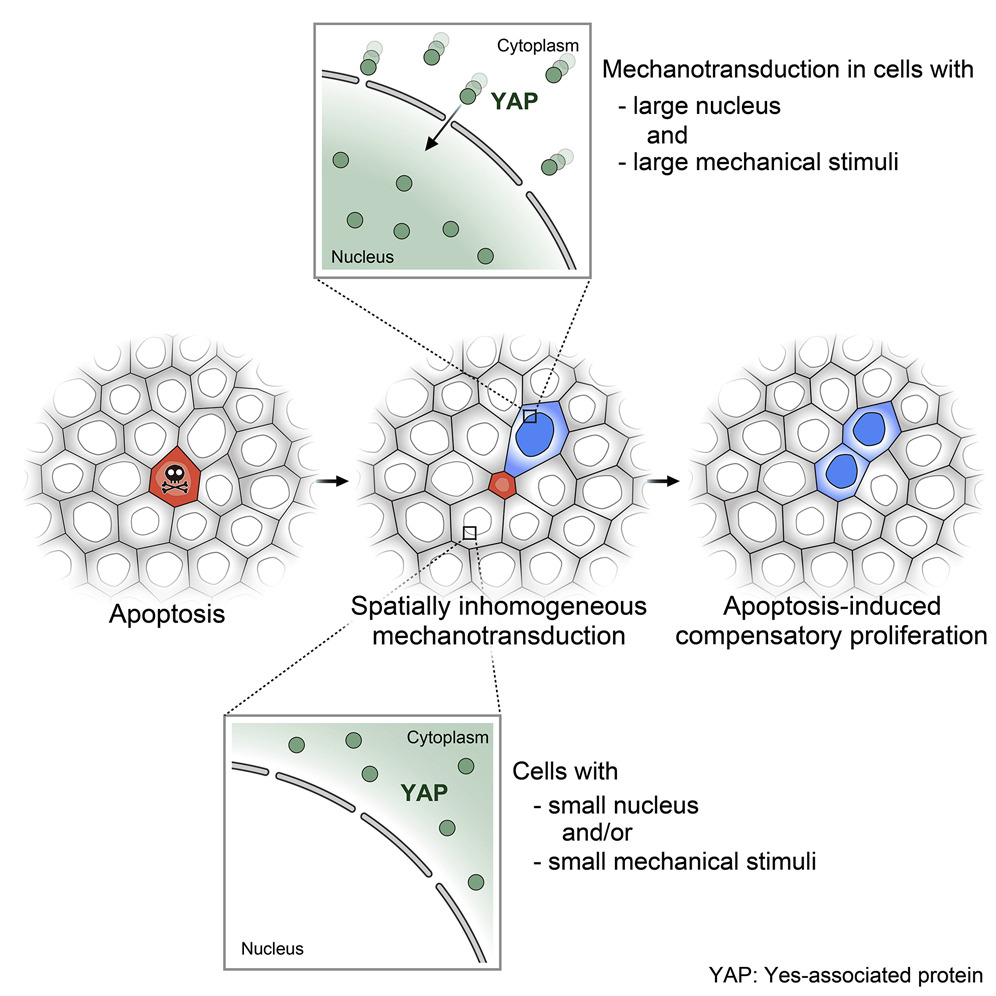
Defining the pattern of cell death and replacement
Researchers from the Toyama Lab at MBI reveal how mechanical factors control apoptosis and cell replacement. https://www.mbi.nus.edu.sg/featured-research/defining-the-pattern-of-cell-death-and-replacement
Bridging the (BP)GAP in metastasis
A collaborative study between researchers at MBI and scientists locally and overseas discovered how a scaffolding protein synchronizes, in space and time, two important regulatory proteins driving cell migration Learn more
Featured Publication
 The Michael Sheetz Lab
The Michael Sheetz Lab
The Sheetz Lab is engaged in studies to understand the detailed molecular mechanisms involved in a variety of phenomena from cancer metastasis to brain function. Learn more.
 The Hanry Yu Lab
The Hanry Yu Lab
The Yu Lab’s research spans from basic biological studies to integrative engineering of biomedical devices that facilitate the translation of systems-level understanding of biological functions into significant applications. Learn more.
The Cell as a Machine
Part of Cambridge Texts in Biomedical Engineering
Published through Cambridge University Press and available in March of 2018, MBI Principal Investigators Michael Sheetz and Hanry Yu have written a unique introductory text explaining cell functions using the engineering principles of robust devices.
Adopting a process-based approach to understanding cell and tissue biology, the book describes the molecular and mechanical features that enable the cell to be robust in operating its various components, and explores the ways in which molecular modules respond to environmental signals to execute complex functions.
Part I. Principle of Complex Function in Robust Machines:
- Robust self-replicating machines shaped by evolution
- Complex functions of robust machines with emergent properties
- Integrated complex functions with dynamic feedback
- Cells exhibit multiple states, each with different functions
- Life at low Reynolds number and the mesoscale leads to stochastic phenomena
Part II. Design and Operation of Complex Functions:
- Engineering lipid bilayers to provide fluid boundaries and mechanical controls
- Membrane trafficking – flow and barriers create asymmetries
- Signaling and cell volume control through ion transport and volume regulators
- Structuring a cell by cytoskeletal filaments
- Moving and maintaining functional assemblies with motors
- Microenvironment controls life, death and regeneration
- Adjusting cell shape and forces with dynamic filament networks
- DNA packaging for information retrieval and propagation
- Transcribing the right information and packaging for delivery
- Turning RNA into functional proteins and removing unwanted proteins
Part III. Coordination of Complex Functions:
- How to approach a coordinated function – cell rigidity sensing and force generation across length scale
- Integration of cellular functions for decision making
- Moving from omnipotency to stable differentiation
- Cancer versus regeneration – the wrong versus right response to the microenvironment.
MBI Video
Hew Choy Leong receives Alumni Award for Academic Achievement
Emeritus Professor Hew Choy Leong, Senior Advisor to MBI, is honoured with an Outstanding Alumni Award for Academic Achievement.
Avery Sun selected for CEMB Future Leaders in Mechanobiology Seminar Series
MBI PhD student Avery Sun was selected for the Future Leaders in Mechanobiology Seminar Series in February 2025. This monthly seminar series is hosted by the Center for Engineering Mechanobiology, University of Pennsylvania, USA.
Hilary Lappin-Scott visiting from Cardiff
Prof. Hilary Lappin-Scottvisiting MBI on 27th November, 2019, for an afternoon of talks, discussion and networking opportunities.
MBI Publications
Latest Publications
- Ong HT, Sriram M, Susapto HH, Li Y, Jiang Y, Voelcker NH, Young JL, Holle AW, and Elnathan R. The Rise of Mechanobiology for Advanced Cell Engineering and Manufacturing. Adv Mater 2025;:e2501640. [PMID: 40576525]
- Lee Y, Shen X, Dreesen O, Zhua J, and Li R. Dysregulation of Extracellular Fibronectin and 5-integrin in Dermal Aging. Mol Biol Cell 2025;:mbcE25020074. [PMID: 40560397]
- Kumari A, Kumar A, Xu G, Roy K, Pratap R, Holle A, and Tijore A. Confinement Suppresses Mechanical Force-Mediated Cancer Cell Apoptosis. Small 2025;:e2504261. [PMID: 40556557]
- Zhang X, Bai J, Fan S, Liang L, Song X, Nai MH, Zhang R, Chen M, Wang J, and Lim CT. Dissecting Exosomal-Tumoral-Vascular Interactions of Single Tumor Cells and Clusters Using a Tumoral-Transendothelial Migration Chip. ACS Nano 2025;. [PMID: 40556461]
- Wang J, Qin Y, Wu Q, Zeng D, Gao X, Wang Q, Li Z, Ni Y, Li H, Zhang P, Guo J, Ma W, Maitusun M, Jin X, Chen M, Zhu L, Lu Q, Chen Q, Wu Y, Lin C, Han R, Cheng D, Ni J, Wang X, Yao F, Zhuang J, Xia J, Liu D, Lu Y, Kang P, Yu S, Chen A, Zhang Y, Li Q, Ge W, Long X, Jiang Z, Guan Z, Jin Z, Jin P, Li T, Shu J, Yang J, Wang J, Jiang N, Qian J, Jung Y, Zhang H, Yang Q, Ma L, Wu X, Huang X, Si D, Ren J, Qiao H, Guo Y, Huang Z, Wang W, Deng W, Bi L, Zhao D, Li Y, Lau RWH, Tham Y, Ma X, Ma J, Shen D, Zhang S, Guan H, Zou W, Guo M, Guan X, Yang X, Xu A, Wu J, Panagiotou G, Tse MA, Kim J, Wu E, Thalmann D, Thalmann NM, Fregni F, Wong TY, Jia W, Zeng R, Lim CT, Sheng B, and Li H. An adaptive AI-based virtual reality sports system for adolescents with excess body weight: a randomized controlled trial. Nat Med 2025;. [PMID: 40551019]
- Tsikolia N, Nguyen DTL, and Tee YH. Mechanisms of left-right symmetry breaking across scales. Curr Opin Cell Biol 2025; 95:102564. [PMID: 40544609]
- Chang Y, Lee JWN, and Holle AW. The mechanobiology of fibroblast activation in disease. APL Bioeng 2025; 9(2):021505. [PMID: 40538750]
- Miotti P, Scarpone M, Lim CT, and Pivkin IV. A Computationally Efficient Viscoelastic Eukaryotic Cell Model. Ann Biomed Eng 2025;. [PMID: 40537593]
- Wang C, Shirzaei Sani E, Shih C, Lim CT, Wang J, Armstrong DG, and Gao W. Wound management materials and technologies from bench to bedside and beyond. Nat Rev Mater 2024; 9(8):550-566. [PMID: 40535534]
- Li X, Wan S, Pronay TS, Yang X, Gao B, and Lim CT. Toward next-generation smart medical care wearables: where microfluidics meet microneedles. Nanoscale Horiz 2025;. [PMID: 40521959]
Biomaterial shows how ageing in the heart could be reversed
A new lab-grown material has revealed that some of the effects of ageing in the heart may be slowed and even reversed. The discovery could open the door to therapies that rejuvenate the heart by changing its cellular environment, rather than focusing on the heart cells themselves.Learn more
Rejuvenation of aged egg cells
MBI researchers collaborated with NUS Bia-Echo Asia Centre for Reproductive Longevity and Equality, based at the NUS Yong Loo Lin School of Medicine, to develop an innovative technique to significantly enhance the reproductive potential of aged oocytes, or immature egg cells, potentially paving the way for better outcomes of assisted reproductive technologies, such as in-vitro fertilisation (IVF), for older females. Learn more
Microtubules and Cell Movement: A Closer Look at Focal Adhesion Disassembly
Researchers from the Bershadsky Lab at MBI utilized optogenetics to unlock the role of microtubules in regulating focal adhesion disassembly, an important step in cell migration. http://www.mbi.nus.edu.sg/featured-research/microtubules-and-cell-movement-a-closer-look-at-focal-adhesion-disassembly