Molecular controllers of stem cell mechanics
How actin regulators interact closely to determine cortex architecture
Written by Sruthi Jagannathan | Microscopy image provided by the Kanchanawong Lab | August 2019
Although it is now known that the mechanical pathways that regulate stem cell functions are different from those operating in specialized cells, the molecular basis for such differences remain unclear. In this study, a team of researchers led by graduate student Shumin Xia and Associate Professor Pakorn Tony Kanchanawong from the Mechanobiology Institute, National University of Singapore, have used super resolution imaging to study the nanoscale organization of the cortical actin cytoskeleton – the filamentous structural framework of the cell that determines its mechanical properties – in mouse embryonic stem cells (mESCs) and understand the role of molecules such as Arp2/3, formins, and capping protein in coordinating cytoskeletal architecture in mESCs.
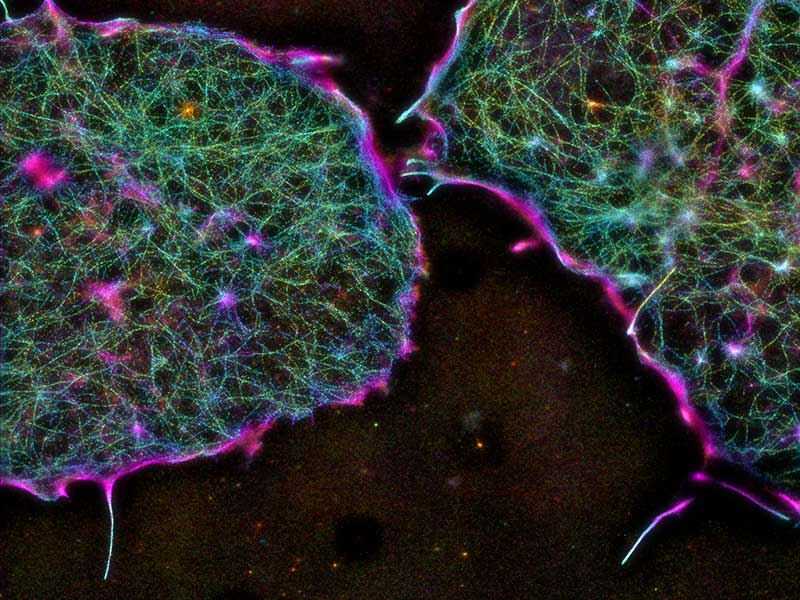
The architecture of the cortical actin cytoskeleton in mouse embryonic stem cells, as imaged by iPALM (interferometric photoactivated localization microscopy).
Aster ‘hubs’ in the cortex control global cortical actin architecture in mESCs
Of the many advances that biomedicine has seen over the past several decades, few have revolutionized the field like stem cell research. The promise of technologies like tissue engineering and regenerative medicine, which employs therapeutic stem cells to create fully functional tissues and organs, has brought hope to millions of patients who suffer from debilitating or life-threatening diseases such as Parkinson’s, Alzheimer’s, organ failures, diabetes, and blood disorders.
Stem cells are unspecialized cells that are mainly characterized by their ability to ‘specialize’ or transform into any cell type in the body. Based on where they are derived from – embryos or adult organisms – they are referred to as embryonic stem cells or adult stem cells.
In order to understand what gives stem cells their unique transformation abilities, scientists have been investigating how the biochemical and mechanical properties of stem cells are different to those of specialized cells. It is now known from such studies that stem cells have a relatively softer nucleus; this helps their chromosomes to flexibly rearrange into specific configurations and customize their gene expression patterns, based on what type of cell they transform into.
By providing insights into the molecular-level mechanisms driving nanoscale organization and dynamics of the actin cortex in mouse embryonic stem cells, findings from the study enhance our understanding of the fundamental principles that govern the mechanics and functions of these therapeutically-important cells.
A soft nucleus needs to be surrounded by a soft cytoplasm, so that internal mechanical homeostasis is maintained in stem cells. This ‘soft’ mechanical state also means that stem cells sense mechanical signals from their environment (such as the architecture and stiffness of the extracellular tissue surrounding them) and transmit it to the nucleus differently, compared to signaling pathways that operate in stiffer specialized cells. Although the existence of altered cell mechanics in stem cells is now well known, how it is ultimately controlled at the molecular level is not clear.
Bridging this knowledge gap was the main idea of the present study carried out by researchers from the Mechanobiology Institute (MBI), National University of Singapore (NUS), along with collaborators from NUS, the South University of Science and Technology of China, and the University of Waterloo, Canada. Led by MBI graduate student Shumin Xia and Associate Professor Pakorn Tony Kanchanawong, the researchers focused on the nanoscale organization of the actin cytoskeleton in mouse embryonic stem cells (mESCs).
They chose to study the actin cytoskeleton as this is the main internal structural framework found in cells. It exists as interconnected filaments of actin proteins, and is, per se, the chief determinant of the mechanical properties of a cell.
By using advanced super-resolution imaging techniques that are capable of dissecting actin network architecture to its finest detail, they found some interesting differences in the organization of actin lying beneath the cell surface (cortical actin) in mESCs. In mESCs, cortical actin was sparse, but uniformly distributed, compared to the more dense and non-uniform organization of cortical actin in specialized cells. Scattered throughout the sparse actin in mESCs were regions of dense filament distribution, known as asters that were surrounded by radial actin filaments growing out of the aster ‘hubs’.
It is now well-known that actin mechanics in specialized cells is mainly controlled by a motor protein called myosin-II. Myosin-II forms bundles that crosslink actin filaments and cause them to slide past each other in contractile motion, resulting in the generation of internal cellular forces. However, the researchers found that mESC cortical actin is sparse enough to exclude myosin II from its organization, and therefore, actin mechanics, in these cells, is largely independent of myosin II functions.
If not myosin-II, what could be controlling actin mechanics in mESCs? The researchers proposed that the sparse, uniform distribution of cortical actin in mESCs arises from a fine balance between the activities of two actin polymerizing proteins – Arp2/3 and formin. The two proteins function in different ways to polymerize actin monomers into longer filaments: while formin generates long, linear actin filaments, Arp2/3 generates branched actin networks. Consistent with this, formin depletion led to more Arp2/3 activity and a denser cortical actin; alternately Arp2/3 depletion led to more formin activity and sparser cortical actin.
They also suggested that asters could serve as ‘hubs’ where Arp2/3 and formin activities are intricately coordinated in order to achieve this fine architectural balance. Operating within this hub is a third protein called capping protein; CP competes with formins in binding to actin filament tips and opposes formin-mediated actin polymerization. Therefore, by indirectly enabling Arp2/3-mediated branched filament organization, CP, along with formins and Arp2/3 plays a critical role in determining cortical actin architecture in embryonic stem cells.
The use of powerful imaging tools in this study has revealed the nanoscale structural organization of the actin cytoskeleton in embryonic stem cells. The detailed, molecular-level insight gleaned from the study has increased our understanding of the fundamental principles governing the mechanics and functions of these therapeutically-important cells. With such information in hand, scientists can hope to create controlled environments for stem cell therapies, under which molecular interactions could be carefully fine-tuned and cortical architecture precisely defined, all of which may improve stem cell transformation efficiencies.