B(a)iled Out
SRUTHI JAGANNATHAN | mAY 2017
A multidisciplinary team of researchers from the Mechanobiology Institute (MBI), National University of Singapore (NUS) have described the mechanical principles adopted by liver cells as they remove excess bile during obstructive cholestasis. This study is published in the Journal of Hepatology [Gupta K, et al. Actomyosin contractility drives bile regurgitation as an early response during obstructive cholestasis. 2017. Doi: 10.1016/j.jhep.2017.01.026. Epub ahead of print].
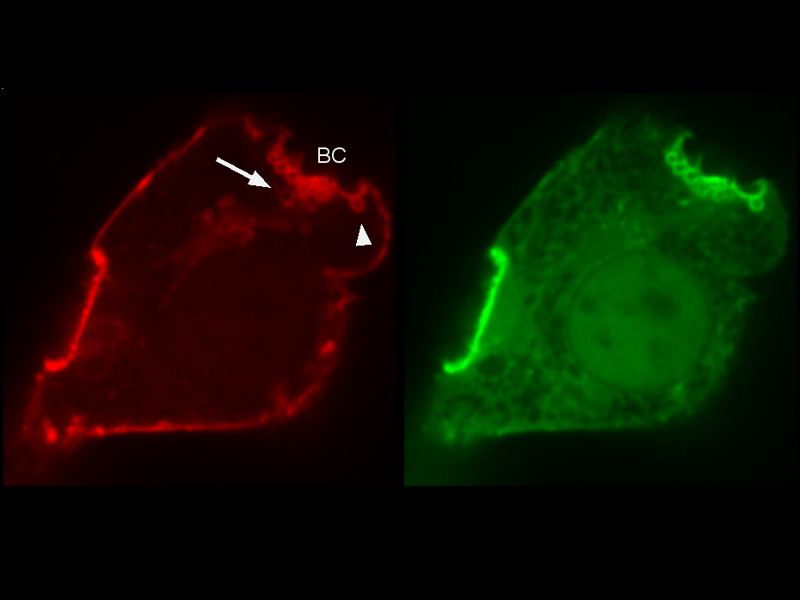
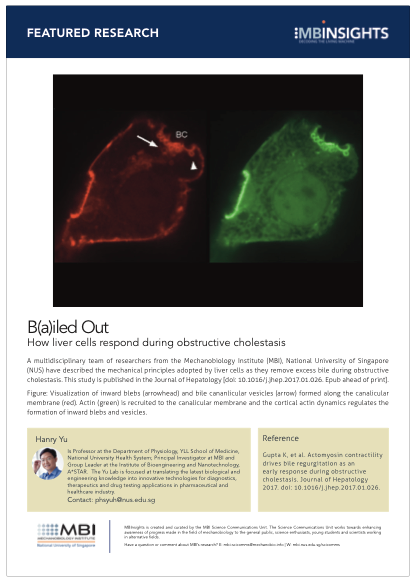
Visualization of inward blebs (arrowhead) and bile cananlicular vesicles (arrow) formed along the canalicular membrane (red). Actin (green) is recruited to the canalicular membrane and the cortical actin dynamics regulates the formation of inward blebs and vesicles.
How liver cells respond during obstructive cholestasis
Dubbed as the ‘chemical factory’ of our body, the liver performs over 500 biological functions. Foremost among them is the synthesis and storage of essential biochemical substances, as well as the elimination or neutralization of harmful toxins from the blood. Bile, which is secreted by liver cells, or hepatocytes, is responsible for the detoxifying effects of the liver. As bile flows through the biliary tract into the small intestine, the bile acids digest fats, and absorbs essential fatty acids, while waste products such as bilirubin in the bile are eliminated via the feces. Therefore, an unimpaired flow of bile fluid is essential for maintaining a state of chemical balance in the body. When this balance is disrupted, toxicity from the accumulating bile may lead to permanent liver damage. The mechanical blockage of bile flow is clinically referred to as obstructive cholestasis, and is known to accompany a number of liver diseases including hepatitis, cirrhosis, and biliary atresia.
The development of improved diagnostic and therapeutic platforms for patients suffering from such liver diseases requires a thorough understanding of the molecular changes that occur in liver cells in response to any disruption in bile flow. In recent years it has become increasingly apparent that a cellular structure found in nearly all cells, the actin cytoskeleton, plays a significant role in regulating bile flow. This structure is made of protein-based filaments and provides structural framework to cells. It also dynamically remodels into higher-order structures in response to mechanical signals, and as it does so, generates forces that are essential to many cellular functions.
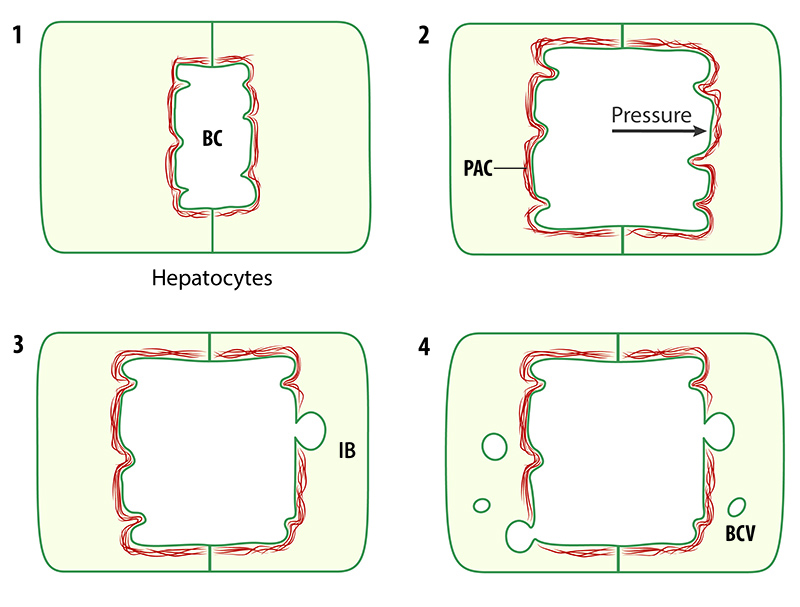
Schematic of events leading to the formation of inward blebs (IB) and bile canalicular vesicles (BCV). When the pressure inside the bile canaliculus (BC) rises due to accumulating bile, the pericanalicular actin cortex (PAC) remodels to induce the formation of blebs and vesicles.
This study demonstrates the major role that cell mechanics plays in clearing away excess bile from the liver in order to maintain a state of cellular homeostasis.
It is one such actin-remodeling event, which occurs around bile canaliculi (small channels formed between adjacent liver cells through which bile is secreted into the bile tract) that has been the focus of a study led by Prof Hanry Yu, who is a Principal Investigator at the MBI and the Institute of Bioengineering and Nanotechnology (IBN), Singapore and MBI graduate student, Kapish Gupta, together with a multidisciplinary team of collaborators from MBI, including Assoc. Prof. Virgile Viasnoff, Assoc. Prof. Low Boon Chuan, and Asst. Prof. Pakorn (Tony) Kanchanawong.
In this work, the research team used an artificial culture system, which allowed the easy manipulation of cultured liver cells. To visualize bile canaliculi dynamics within these cells, high-end imaging techniques were employed, with the researchers observing the accumulation of a thick actin network, called the pericanalicular actin cortex (PAC), around the bile canaliculi. To investigate the cellular response to a blocked bile duct, the team obstructed the bile tract artificially. This led to the discovery that the bile canalicular surface protruded inwardly at several points, forming structures that the team referred to as inward blebs. The formation of these inward blebs was linked to increased pressure inside the obstructed bile tract, which caused the actin network to rupture at certain points, thereby releasing the canalicular surface, and generating the inward protrusions.
In most cases these inward blebs were short-lived, and they soon retracted as the actin cortex was repaired, or resealed, by protein-based machines. However, when the pressure inside the canaliculus reached a certain level, the actin repair mechanisms could not generate sufficient force to retract the blebs that had developed. Ultimately, this led to the formation of bubble-like vesicles, known as bile canalicular vesicles (BCV), within the cytoplasm of liver cells.

MBI Graduate Student Kapish Gupta in the MBI labs
Importantly, BCV formation is physiologically important, as it is strongly correlated with bile acid clearance from the canaliculus when the bile flow is otherwise blocked. During conditions such as obstructive cholestasis, when the pressure in the canaliculus rises due to the accumulation of bile, the liver cells will use these vesicles as an early response aimed at clearing away or ‘regurgitating’ excess bile from the liver.
This study demonstrates the major role that cell mechanics plays in clearing away excess bile from the liver in order to maintain a state of cellular homeostasis. During obstructive cholestasis, when bile accumulation leads to increased pressure in the canaliculus, the actin cortex surrounding the canaliculus (the PAC) remodels to induce the formation of vesicles that serve as carriers to transport excess bile out of the liver. These findings may have promising clinical applications. The pericanalicular actin cortex may, for example, serve as a potential target for therapeutics aimed at treating life threating liver diseases. It is hoped that in targeting the pericanalicular actin cortex, the rate of vesicle formation and thus, bile regurgitation, can be controlled in a number of liver diseases including bile atresia, which is a life-threating liver disease most commonly affecting Asian infants.