Understanding how cells sense and interact with their environment
Cells communicate with their immediate environment through contact points called ‘cell adhesions’. Cells can form adhesion complexes with neighboring cells or with the extracellular matrix, and in both cases, these adhesion points exist as modular units containing multiple proteins and cytoskeletal assemblies.
MBI researchers have revealed the underlying mechanism for the formation and growth of a fundamental type of tissue–epithelial tubes. Read the full article: Shaping Lumens by Force.
Adhesions that connect the cell with its immediate environment, the extracellular matrix, are most commonly formed around a protein known as integrin, and are called ‘focal adhesions’. At the MBI, we seek to dissect the molecular composition, nanoscale structure, dynamic organization and mechanosensing functions of integrin-based focal adhesions. In particular, we are interested in the role of focal adhesions in cancer cells, where dysregulation in the formation and functions of focal adhesions is associated with increased motility of cancer cells.
Cells also communicate with each other
Eukaryotic cells rarely exist in isolation from other cells, and their ability to exist in larger communities is imperative to tissue development and integrity. Cell-cell adhesions serve as the structural backbone connecting neighboring cells, and being connected internally to the cellular cytoskeleton, these complexes are also crucial in the transduction of mechanical signals generated at the tissue level.
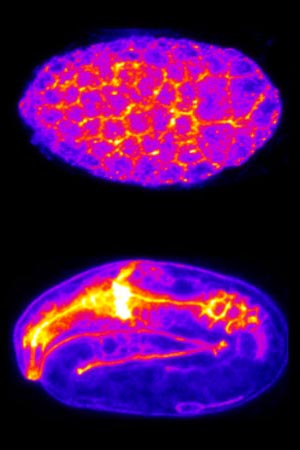
An MBI study reveals that glycolipids which anchor certain proteins to the cell membrane also play a vital role in stabilizing the membrane. Read the full article: Lipid Anchors Sustain Life.
Of the various types of adhesive structures line the lateral membranes of polarized cells, cadherin-based adherens junctions, and claudin-based tight junctions, are the most commonly studied.
At MBI, we are working towards characterizing the molecular architecture, assembly, and mechanosensory function of adherens junctions. Our work has led to the description of a cadhesome complex at adherens junctions, which brings together more than 500 proteins through dynamic protein-protein interactions, highlighting the significance of adherens junction in cell signaling and multicellular organization.
Another major area of our research is directed at studying the underlying principles in force generation that are essential for the coordinated movement of epithelial cells during tissue repair mechanisms. By employing state-of-the-art micro- and nanofabrication tools to control the mechanical properties of cell’s microenvironment, we are gaining greater insight on the role of cell-cell signaling in the regulation of cytoskeletal remodeling, and hence the cellular response to changes in the microenvironment.
Finally, the role of cell-cell adhesions in the regulation of signaling pathways is also under investigation. For example, the role of the zona occludens (ZO) proteins, including ZO-1 and ZO-2, in the tumor suppressor pathways such as the Hippo signaling pathway.
Understanding membrane biophysics
We are also exploring the dynamics of the cell membrane in order to understand how his structure enables the various mechanosensitive properties of cell adhesions, and how the dynamic remodeling of the membrane facilitates the formation and function of motile structures.
In particular, the role of the physico-chemical properties of the membrane, such as membrane curvature and protein-lipid interactions, and its dynamic crosstalk with the cytoskeleton, in regulating vital cellular processes are some aspects currently under investigation.


