More twists to the tale
Varied actin patterns in epithelial cells
Written by Sruthi Jagannathan | Microscopy images from the Bershadsky Lab | July 2019
How do actin organization patterns differ between cell types and how does this play a role in determining tissue- and organ-level asymmetries during development? A recent study from the Bershadsky Lab at the Mechanobiology Institute, National University of Singapore has revealed the different actin organization patterns (in particular, observing the presence or absence of swirling actin filaments) in fibroblasts and epithelial cell subtypes, and identified mDia1 as the molecular regulator that is responsible for the chiral swirling of actin filaments in single cells, which in turn, results in tissue-level left-right asymmetries. The study was published in the Journal of Cell Science.

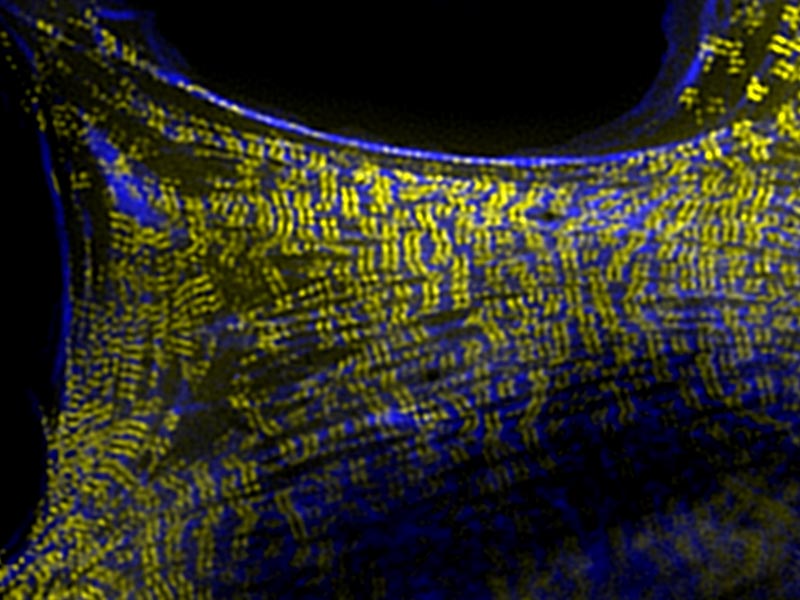
Radial (left) and chiral (right) patterns of organization of the actin cytoskeleton (magenta and cyan) and keratin network (yellow and red) in keratinocytes. Chiral actin and keratin organization in keratinocytes was induced by treatment with low doses of latrunculin A. A similar artistic compilation of the microscopic images was featured as the cover image for the March issue of the Journal of Cell Science.
Actin self-organization differs in fibroblasts and epithelial cells
Have you ever wondered why your heart lies to the left or why your liver is positioned on the right side of your body? The asymmetric positioning of some of our internal organs either to the right or left is not a random occurrence, but an evolutionarily conserved process, known to go awry only rarely in individuals with a congenital anomaly called situs inversus, who have flipped or mirrored positioning of their internal organs. A robust decision making mechanism must therefore exist to help tissues and organs discern between left and right, as early as during embryo development, when millions of cells are coming together in a highly orchestrated fashion to form future tissues and organs of our body.
This led scientists to speculate that single cells must possess intrinsic asymmetry, in order to be able to drive organ-level patterning during development. For several years, they looked for changes occurring within single cells that may cause them to assume such left-right asymmetry. Not long ago, researchers from the Bershadsky Lab at the Mechanobiology Institute, National University of Singapore, ventured a step further: they looked for clues to the origin of this left-right decision making process beyond the cellular level – within subcellular structures and molecules that are found in a cell.
Actin swirling induces asymmetry in single cells
The researchers focused on the behavior of actin cytoskeleton, which exists as a subcellular structural framework made of interconnected filaments of individual actin protein subunits. In an earlier study, they grew fibroblasts (a type of cell found in the connective tissue that has a well-developed actin cytoskeleton) on standardized circular patterns, on which innate actin behavior can be studied.
By growing cells on standardized circular patterns, researchers from the Bershadsky Lab observed differences in actin organization patterns between fibroblasts and epithelial cell sub types.
When actin behavior differs between cell types
Following on from this earlier work, MBI graduate student Salma Jalal and Senior Research Fellow Dr Yee Han Tee from the Bershadsky Lab employed similar standardized circular patterns to examine actin organization in different subtypes of epithelial cells (cells lining the surfaces of organs), in addition to fibroblasts.
Interestingly, they found some important deviations in actin behavior in epithelial cells compared to fibroblasts. While, in fibroblasts, actin filaments transitioned through the expected circular, radial, and swirling patterns (as described in their previous study), in epithelial cells they assembled just into circular patterns (in non-skin epithelial cells), or proceeded to form radial patterns (in skin epithelial cells) at most. Importantly, actin did not form asymmetric, swirling patterns in either subtype of epithelial cells. However, by treating the skin epithelial cells with latrunculin A – a drug that affects actin polymerization by sequestering actin monomers – the researchers were able to induce swirling of the actin filaments in these cells.
Formins as the molecular drivers of actin swirling
One key question that boggled the researchers was what could be causing the swirling of the actin cytoskeleton in these cells? To find answers, they dug deeper at the molecular level and identified a protein named mDia1 (DIAPH1) as the key molecular driver of left-right asymmetry. mDia1 (a member of the formin family of actin regulatory proteins) facilitates the assembly of individual actin monomers into long filaments that comprise the radial fibers. The spiraling of newly added monomers around the axis of the growing filament generates a left-right rotational torque; the mechanical twist induced by formin-driven actin polymerization could result in swirling of the radial fibers. The researchers also found that the swirling of the actin cytoskeleton drives the rotation of other subcellular structures, including the nucleus and the keratin intermediate filament network, in the same direction.
The researchers propose that a similar molecular mechanism could be the underlying basis for the origin of asymmetry in other biological systems too. In fact, recent studies have reported the role of a formin gene in determining left-right asymmetry during snail embryonic development (Davison 2016; Kuroda 2016 and 2019).
In recent years, an increasing number of studies are adopting a bottom-up approach for unraveling the basic details of a biological process. This study, using such an approach, offers key insights into the subcellular and molecular mechanisms that could confer single cell-level asymmetries and set into motion a complex cascade of events that would lead to organism-level asymmetries in biological systems.