Unraveling α-catenin
Steven J Wolf | August 2014
MBI researchers, in collaboration with researchers from the Institute Jacques Monod (IJM) (Paris Diderot University and CNRS, France), have discovered how α-catenin confers mechanosensing properties to cell-cell junctions.
This work was published in Nature Communications (Yao M, et al. Force dependent conformational switch of a-catenin controls vinculin binding. Nat Commun. 2014. doi:10.1038/ncomms5525).
α-catenin mediated mechanosensing of cell-cell junctions
Proteins are molecular tools responsible for carrying out a multitude of fundamental processes within the cell. The specific function of a protein is closely linked to its physical properties. These include size, shape and chemistry. This is similar to the tools we use every day; the shape of a spanner, for example, makes it ideal for tightening bolts. Like the tools in a toolbox, the diversity in protein size, shape and structure mean that each type of protein is responsible for executing a specific task. Some proteins are suited to providing structural support to the cell while others can initiate biochemical reactions or transport molecular cargo. Some can even detect and transduce mechanical force.
How can proteins detect force?
The physical and chemical properties of a protein are determined by the precise folding of the amino acid chain, which is the basic molecular backbone of all proteins. Although a protein’s role may be reflected in its structure or shape, functionally important regions are often concealed deep inside the protein. These regions are only exposed when the protein changes its shape, or adopts another conformation, in response to chemical or mechanical cues. In some cases this will see the protein unfold, and refold, particular regions.
This phenomenon was the focus of a recent collaborative study led by Profs Yan and Ladoux from the Mechanobiology Institute, Singapore and Prof Mège of the Institute Jacques Monod, CNRS, France. In their study, the structural properties of α-catenin were explored and its role as a mechanosensor quantitatively established for the first time.
The findings presented by Yao et al advance our understanding of how cells adapt and respond to mechanical forces from neighboring cells.
In cells, α-catenin exists as an integral component of cell- cell adhesion complexes. Specifically, α-catenin connects membrane bound proteins that interact with neighboring cells to a network of filaments that lay adjacent to the cell membrane, known as the actin cytoskeleton. Although α-catenin was long suspected to provide adhesion complexes with the ability to detect mechanical force, no conclusive evidence had been obtained to confirm how this occurred.
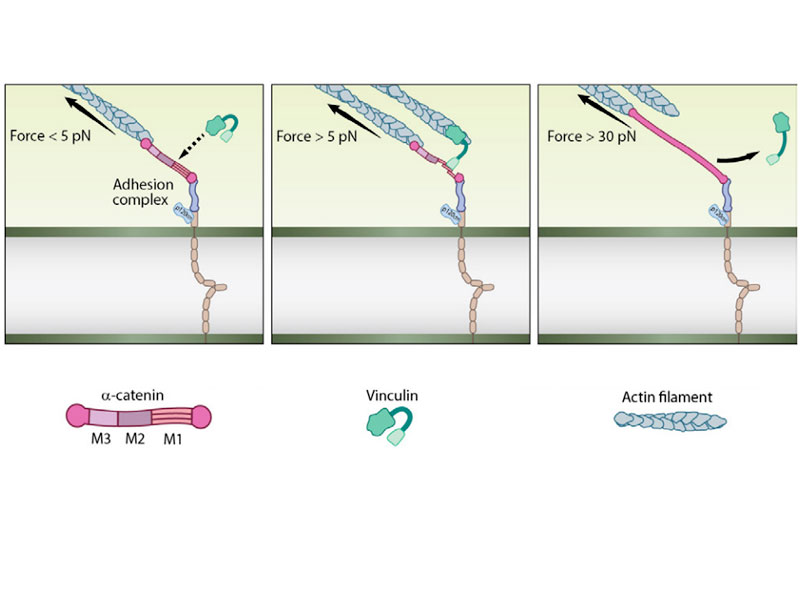
Using magnetic tweezers to stretch single α-catenin molecules in vitro, Yao et al were able to assess which regions of the protein unfolded, and which were functionally relevant. Stretching α-catenin with around 5 pN of force caused an unfolding event that exposed a single vinculin-binding site within α-catenin. With vinculin bound, the protein remained unfolded, or extended, even when the stretching force was released.
However, when a much larger force was applied (>30pN), vinculin detached from a-catenin and the protein subsequently refolded into its more compact shape. Both 5 pN and 30 pN are physiologically relevant forces. The former could be generated by a single or a few myosin molecules pulling on actin filaments while the latter could be produced from the larger actomyosin network. Such a mechanism of unfolding, stabilization and refolding is similar to that which occurs when a foldable table is opened. Pulling on the table will unfold the table top. However the table will refold unless a safety latch is used to lock the table in the open position. Once this latch is in place, the table can be used without it collapsing. With additional extension however, the safety lock can disengage, and the table top will fold back up to its compact shape.
Cell-cell adhesions are crucial in the organization, maintenance and repair of multicellular structures such as tissues and organs. With α-catenin playing an equally integral role in the overall function of these adhesions, the findings presented by Yao et al advance our understanding of how cells adapt and respond to mechanical forces from neighboring cells. This is crucial in determining how cell- cell adhesions are disrupted in disease states such as cancer, where a loss of adhesion is a prerequisite for cellular invasion.