A Cellular Shock Absorber
Andrew MS Wong and Lakshmi Ramachandran | July 2016
A research team from the Mechanobiology Institute (MBI), National University of Singapore, and the University of Kent and University of Cambridge in the UK, show how a protein called talin senses mechanical force exerted on a cell and buffers the force through stochastic unfolding and refolding of its multiple folded domains. This phenomenon (or role of talin) defines the range of force that can be stably transmitted through the cell, and is therefore crucial in the regulation of many cellular processes. This study is published in Nature Communications (Yao M, Goult BT et al, The mechanical response of talin, Nat Comms, 7:11966, doi: 10.1038/ncomms11966).
How talin structure makes it an ideal force buffer
In the body, cells are continuously impacted by mechanical forces. These forces can arise from the external surroundings or are generated internally by dynamic contractions of the cytoskeleton, a filamentous protein network within the cell. Maintaining and balancing a certain level of force is essential –too much force can lead to physical disruption of the mechanical linkages in the cell, but insufficient force will lead to inactivation of mechanical signalling pathways necessary for cell movement and development.
By combining single molecule manipulation experiments with structural biology, physical modelling, and numerical simulation, a collaborative research team led by Assoc. Prof. Jie Yan (MBI & Department of Physics, NUS) and Prof. Michael Sheetz (MBI, NUS) identified that the protein talin is able to buffer force, effectively acting as a shock absorber for the cell.
Talin buffers cellular forces
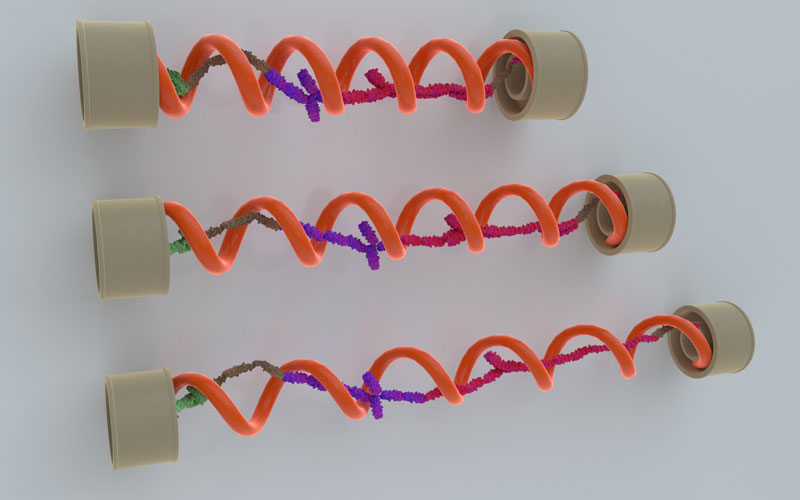
Talin links focal adhesions – protein complexes that connect to the external environment – with the cytoskeleton, which provides structural stability and the basis for cell movement. Previous studies have demonstrated that talin is ‘mechanosensitive’, meaning that it can sense and respond to mechanical force, and convert these forces into biological signals. Structurally, talin has a rod-like shape, subdivided into a linear array of 13 domains, which can unfold to allow it to increase in length several fold. In living cells, the level of force in talin was recently measured to be between 5-10 pN, a range which allows various crucial mechanosensitive protein-protein interactions take place; however, the mechanism of how this force level is maintained was unknown.
Talin is a highly effective force buffer which functions as a cellular shock absorber.
In order to solve this mystery, the scientists used magnetic tweezers to probe the mechanical response of talin at a single-molecule level. The magnetic tweezers were used to apply mechanical force to a single talin molecule, causing the domains inside it to unfold. By carefully measuring the rates of unfolding and refolding of the domains under different forces, the researchers were able to simulate the force fluctuation during the process of stretching and relaxation of talin. They discovered that the average force remains within a narrow range between 5-10 pN, even when talin is fully extended to a length of 500 nm. This result robustly explains the recently reported levels of force measured in talin in living cells. Importantly, this force range defines the force threshold for talin dependent mechanosignalling, a critical pathway that drives a number of biological processes, such as maintaining adhesion stability, growth, and maturation.
This study revealed that talin is a highly effective force buffer which functions as a cellular shock absorber. The physical principle revealed for talin as a force buffer is universal to any protein that is comprised of a linear array of domains. Interestingly, a set of similar proteins have evolved that link the cytoskeleton not only to focal adhesions but also to neighbouring cells and the nucleus. The mechanism revealed in this study therefore suggests that all these proteins can buffer force in a certain range to enable robust mechanosensitive interactions. In a similar way to how the suspension of a car connects the tyres to the chassis and enables a smooth ride even on difficult terrain, these proteins connect adhesions to the cell skeleton and absorb force so that the cell remains mechanically stable and active.