Molecular Mechanobiology
Molecular machines sense and transduce mechanical force
Proteins that are activated or suppressed by mechanical force allow eukaryotic cells, such as those that make up the human body, to respond to changes in their microenvironment. This is crucial during development, when cell niches are still forming. Similarly, the changes in our bodies that occur as we age can lead to an altered physical environment for our cells – for example, bones may become softer, and scar tissue can build up.
Through this work, the importance of mechanics in the regulation of the genome is becoming evident.
By manipulating specific proteins and subjecting cells to a level of physical force consistent with that which occurs naturally in the human body, we are gaining a better understanding of how various molecular machines detect and transduce physical forces. Of particular interest are contractile cytoskeletal units, cadherin-based adhesion complexes, which connect cells with one another, and integrin-based adhesion complexes, which enable cellular interaction with the extracellular matrix.
Several proteins found in these machines can be stretched, thereby exposing domains that will bind additional proteins. This mechanism is replicated in MBI laboratories at the level of single proteins. Understanding the forces required to stretch a protein, to trigger contraction of an actomyosin based complex, or to induce a biochemical change in a protein provides crucial information on how cells integrate physical force into biochemical pathways.
Physical forces are integrated into biochemical pathways to impact genome regulation
Signals established at the cell periphery, or within the cytoskeleton, may converge on the cell nucleus where they will impact genome regulation and protein expression.
At the MBI, we investigate how mechanical cues regulate key signaling pathways, such as the Hippo pathway, and subsequently, the activity of various transcription machinery. In these cases physical cues, which may generated through the contraction or stretching of a protein complex, can lead to the recruitment or activation of other enzymes and transcription factors. Physical force is subsequently converted to a biochemical signal.
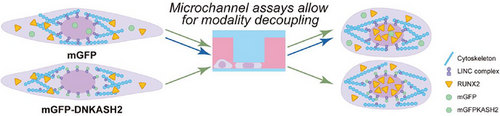
Alternatively, mechanical signals may be transferred through the cell as a physical force. For example, when the cytoskeleton contracts, or when an alteration in cell morphology generates tension within the nuclear membrane, the forces involved are transferred throughout the cell. The effect of transferring force through structural elements of the cell is investigated at the MBI. For example, we grow cells on substrates of a defined shape and monitor how nuclear morphology is affected, and in turn, how this phenomenon alters DNA packaging and the spatial arrangement of genes. Through this work, the importance of mechanics in the regulation of the genome is becoming evident.
The biophysics of bacterial pathogenesis
Like their larger multicellular hosts, bacteria and other prokaryotic cells are subjected to, and generate, mechanical forces. When invading a host cell, forces must be overcome, and in some cases, the dynamic structures of the host cell may be exploited by bacteria to facilitate their invasion.
For example, bacteria can modulate the cell membrane and manipulate the cytoskeleton for entry into the host, or for the secretion of virulence factors. In doing so the bacteria are able to establish an infection. However, the precise molecular mechanisms underlying bacterial uptake, and the mechanisms by which bacteria can evade the body’s defenses remain unclear. At MBI, we are investigating the biophysical aspects of bacterial pathogenesis, and in particular, are interested in the mechanisms by which bacteria like Salmonella utilize the endocytic membrane trafficking pathway to survive within the host. In this case, Salmonella will survive within an endosomal compartment called the salmonella containing vacuole (SCV). Salmonella also thrives within its host in a non-infectious phase for an extended period of time. Here, the bacteria will exist as a biofilm. How salmonella lives within SCVs, the various cytoskeletal modifications that take place during invasion and the regulatory mechanisms that are modulated to enable Salmonella to exist as a biofilm, are all researched at MBI.
Additional work in this area is looking at the formation and nature of cytoskeletal pedestals, which, in the case Enteropathogenic E.coli (EPEC) infection, are found within host cells at the site of bacterial attachment.
Confined Migration Shapes the Bony Fate of Stem Cells
Researchers from the Holle Lab at the Mechanobiology Institute, NUS discover that migration through confined spaces causes lasting nuclear changes in stem cells, biasing them toward bone formation.
Disrupted Cell-Matrix Interactions Drives Aging and Reveals New Paths for Skin Regeneration
Researchers from the Li Lab discover how α5-integrin–FN interactions preserve dermal integrity, offering new insights for antiaging strategies targeting ECM organization and fibroblast function.
DECIPHERing the Role of Cell-Matrix Interactions in Ageing Heart Health
Researchers from the Soft Nano-Biomaterials Lab at MBI developed a material system to enable precise investigation into how individual ECM properties affect cultured heart cells.
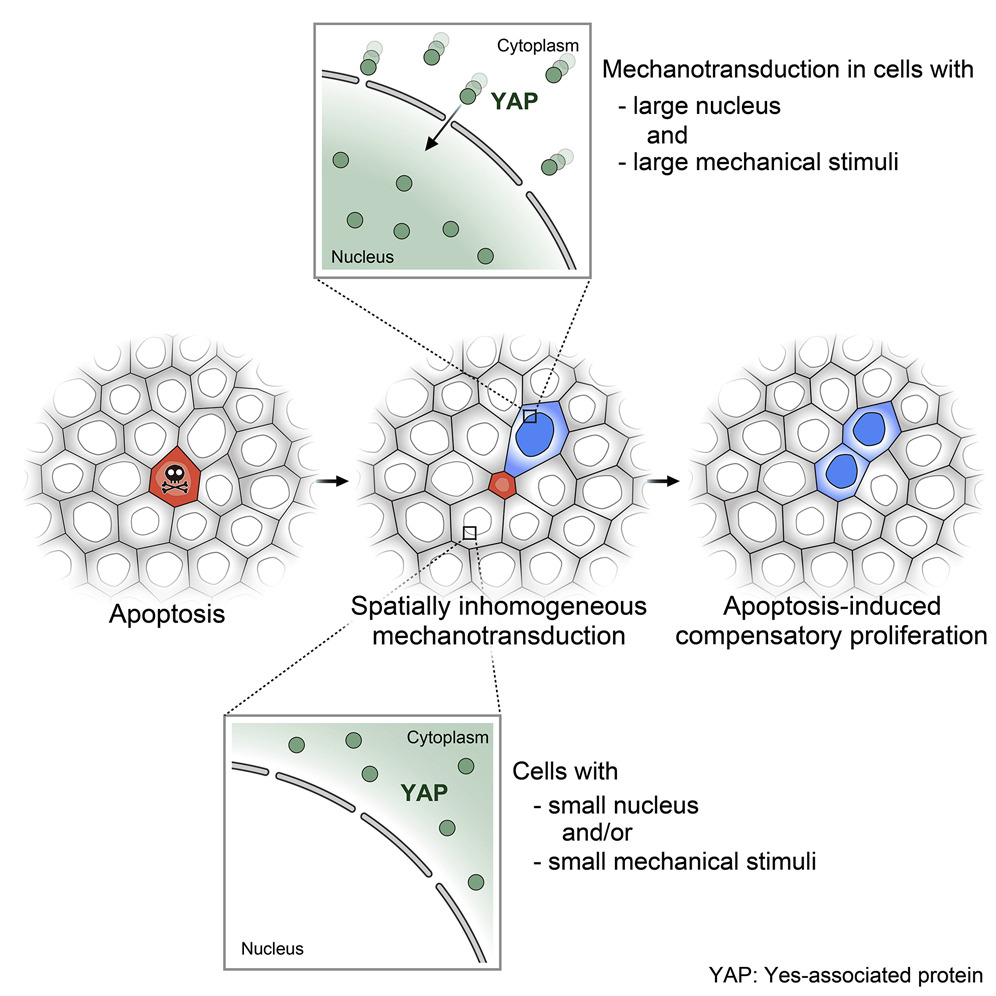
How Dying Cells Signal Growth: Mechanical Forces Drive Targeted Cell Proliferation after Apoptosis
Researchers from the Toyama Lab at MBI reveal how mechanical factors control apoptosis and cell replacement.
Scientists Develop AI-Driven Tool to Map 3D Cellular Landscapes
Researchers from the Beghin Lab at the Mechanobiology Institute, NUS combine DeepStar3D algorithm and 3DCellScope interface to develop an experimental tool for real-time interaction with cell data.
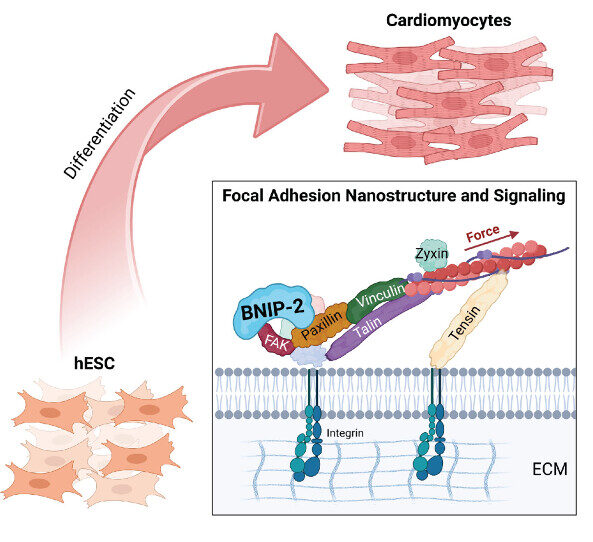
The Nano-Heartbuilder: BNIP-2 Influences Mechanosensing in Cardiomyoblast Differentiation
Researchers from the Low Lab at MBI discover a crucial role for the scaffold protein BNIP-2 in orchestrating focal adhesion dynamics during early heart development, offering new insights into heart regeneration strategies.
Shedding Light on Local Microtubule Regulation of Focal Adhesions
Researchers from the Bershadsky Lab at MBI utilized optogenetics to unlock the role of microtubules in regulating focal adhesion disassembly, an important step in cell migration. http://www.mbi.nus.edu.sg/featured-research/microtubules-and-cell-movement-a-closer-look-at-focal-adhesion-disassembly
Defining the pattern of cell death and replacement
Researchers from the Toyama Lab at MBI reveal how mechanical factors control apoptosis and cell replacement. https://www.mbi.nus.edu.sg/featured-research/defining-the-pattern-of-cell-death-and-replacement
Pakorn Tony KANCHANAWONG
biekp@nus.edu.sg
Super-resolution microscopy, integrin-mediated cell adhesions, nanoscale architecture of cellular structures, more
Linda J KENNEY
kenneyl@uic.edu
Signal transduction in bacteria, bacterial pathogenesis, mechanotransduction and osmotic signaling in E. coli, mechanisms of anti-silencing of virulence genes in Salmonella, more
KOH Cheng Gee
cgkoh@ntu.edu.sg
Cell signaling, regulation of actin cytoskeleton, Rho GTPases their effectors and regulators, more
LOW Boon Chuan
dbslowbc@nus.edu.sg
Cell signaling, domain-discovery, protein-protein interaction, structural biology, developmental biology, computational biology and mechanobiology, more
Publications by PI
- Nishimura R, Barnett SFH, Jain K, Huang Z, Goult BT, and Kanchanawong P. Optogenetic control of mechanotransduction based on light-induced homodimerization of talin. J Cell Sci 2025;. [PMID: 41277405]
- Nishimura R, and Kanchanawong P. Nanoscale mechano-adaption of integrin-based cell adhesions: New tools and techniques lead the way. Curr Opin Cell Biol 2025; 94:102509. [PMID: 40188780]
- Wang W, Huang Z, Wang Y, Li H, and Kanchanawong P. Vortex Interference Enables Optimal 3D Interferometric Nanoscopy. Phys Rev Lett 2025; 134(7):073802. [PMID: 40053988]
- Liu S, Meng Y, Lan X, Li R, and Kanchanawong P. Ground-state pluripotent stem cells are characterized by Rac1-dependent Cadherin-enriched F-actin Complexes. J Cell Sci 2025;. [PMID: 39886806]
- Xiao J, Ang JW, Zhong X, Wong DCP, T T, Yow I, Lee CJM, Foo RS, Kanchanawong P, and Low BC. Coordination of Focal Adhesion Nanoarchitecture and Dynamics in Mechanosensing for Cardiomyoblast Differentiation. ACS Appl Mater Interfaces 2025;. [PMID: 39778877]
- Jain K, Kishan K, Minhaj RF, Kanchanawong P, Sheetz MP, and Changede R. Immobile Integrin Signaling Transit and Relay Nodes Organize Mechanosignaling through Force-Dependent Phosphorylation in Focal Adhesions. ACS Nano 2025;. [PMID: 39760672]
- Lin K, Gujar MR, Lin J, Ding WY, Huang J, Gao Y, Tan YS, Teng X, Christine LSL, Kanchanawong P, Toyama Y, and Wang H. Astrocytes control quiescent NSC reactivation via GPCR signaling-mediated F-actin remodeling. Sci Adv 2024; 10(30):eadl4694. [PMID: 39047090]
- Morales-Camilo N, Liu J, Ramírez MJ, Canales-Salgado P, Alegría JJ, Liu X, Ong HT, Barrera NP, Fierro A, Toyama Y, Goult BT, Wang Y, Meng Y, Nishimura R, Fong-Ngern K, Low CSL, Kanchanawong P, Yan J, Ravasio A, and Bertocchi C. Alternative molecular mechanisms for force transmission at adherens junctions via β-catenin-vinculin interaction. Nat Commun 2024; 15(1):5608. [PMID: 38969637]
- Aureille J, Prabhu SS, Barnett SF, Farrugia AJ, Arnal I, Lafanechère L, Low BC, Kanchanawong P, Mogilner A, and Bershadsky AD. Focal adhesions are controlled by microtubules through local contractility regulation. EMBO J 2024;. [PMID: 38769437]
- Jain K, Minhaj RF, Kanchanawong P, Sheetz MP, and Changede R. Nano-clusters of ligand-activated integrins organize immobile, signalling active, nano-clusters of phosphorylated FAK required for mechanosignaling in focal adhesions. bioRxiv 2024;. [PMID: 38464288]
- Fernandez M, Yamanaka Y, Zangoui P, White MA, and Kenney LJ. The sulfur assimilation pathway mitigates redox stress from acidic pH in Salmonella Typhi H58. mBio 2025;:e0046725. [PMID: 40422406]
- Shetty D, and Kenney LJ. A pH-sensitive switch activates virulence in Salmonella. Elife 2023; 12. [PMID: 37706506]
- Oh D, Liu X, Sheetz MP, and Kenney LJ. Small, Dynamic Clusters of Tir-Intimin Seed Actin Polymerization. Small 2023;:e2302580. [PMID: 37649226]
- Mon KKZ, Si Z, Chan-Park MB, and Kenney LJ. Polyimidazolium Protects against an Invasive Clinical Isolate of Salmonella Typhimurium. Antimicrob Agents Chemother 2022;:e0059722. [PMID: 36094258]
- Jacob H, Geng H, Shetty D, Halow N, Kenney LJ, and Nakano MM. Distinct Interaction Mechanism of RNAP and ResD and Distal Subsites for Transcription Activation of Nitrite Reductase in Bacillus subtilisψ. J Bacteriol 2021;:JB0043221. [PMID: 34898263]
- Singh MK, Zangoui P, Yamanaka Y, and Kenney LJ. Genetic code expansion enables visualization of Salmonella type three secretion system components and secreted effectors. Elife 2021; 10. [PMID: 34061032]
- Kenney LJ, and Anand GS. EnvZ/OmpR Two-Component Signaling: An Archetype System That Can Function Noncanonically. EcoSal Plus 2020; 9(1). [PMID: 32003321]
- Desai SK, and Kenney LJ. Switching Lifestyles Is an in vivo Adaptive Strategy of Bacterial Pathogens. Front Cell Infect Microbiol 2019; 9:421. [PMID: 31921700]
- Desai SK, Padmanabhan A, Harshe S, Zaidel-Bar R, and Kenney LJ. Salmonella biofilms program innate immunity for persistence in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2019;. [PMID: 31160462]
- Ottemann KM, and Kenney LJ. Editorial overview: Host-pathogen interactions: bacteria. Curr. Opin. Microbiol. 2019; 47:iii-v. [PMID: 31138403]
- Zhang S, Chong LH, Woon JYX, Chua TX, Cheruba E, Yip AK, Li H, Chiam K, and Koh C. Zyxin regulates embryonic stem cell fate by modulating mechanical and biochemical signaling interface. Commun Biol 2023; 6(1):62. [PMID: 36653484]
- Wong DCP, Pan CQ, Er SY, Thivakar T, Rachel TZY, Seah SH, Chua PJ, Jiang T, Chew TW, Chaudhuri PK, Mukherjee S, Salim A, Aye TA, Koh CG, Lim CT, Tan PH, Bay BH, Ridley AJ, and Low BC. The Scaffold RhoGAP Protein ARHGAP8/ BPGAP1 Synchronizes Rac and Rho Signaling to Facilitate Cell Migration. Mol Biol Cell 2023;:mbcE21030099. [PMID: 36598812]
- Yip AK, Zhang S, Chong LH, Cheruba E, Woon JYX, Chua TX, Goh CJH, Yang H, Tay CY, Koh C, and Chiam K. Zyxin Is Involved in Fibroblast Rigidity Sensing and Durotaxis. Front Cell Dev Biol 2021; 9:735298. [PMID: 34869319]
- Sathe SR, Jain D, Koh C, and Yim EKF. POPX2 phosphatase enhances topographical contact guidance for cell morphology and migration. Biomed Mater 2020;. [PMID: 33321483]
- Koon YL, Zhang S, Rahmat MB, Koh CG, and Chiam K. Enhanced Delta-Notch Lateral Inhibition Model Incorporating Intracellular Notch Heterogeneity and Tension-Dependent Rate of Delta-Notch Binding that Reproduces Sprouting Angiogenesis Patterns. Sci Rep 2018; 8(1):9519. [PMID: 29934586]
- Ou S, Tan M, Weng T, Li H, and Koh C. LIM kinase1 regulates mitotic centrosome integrity via its activity on dynein light intermediate chains. Open Biol 2018; 8(6). [PMID: 29925632]
- Weng T, and Koh C. POPX2 phosphatase regulates apoptosis through the TAK1-IKK-NF-κB pathway. Cell Death Dis 2017; 8(9):e3051. [PMID: 28906490]
- Zhang S, Weng T, Cheruba E, Guo T, Chan H, Sze SK, and Koh C. Phosphatase POPX2 Exhibits Dual Regulatory Functions in Cancer Metastasis. J. Proteome Res. 2016;. [PMID: 27976581]
- Hoon JL, Tan MH, and Koh C. The Regulation of Cellular Responses to Mechanical Cues by Rho GTPases. Cells 2016; 5(2). [PMID: 27058559]
- Khaw S, Min-Wen C, Koh C, Lim B, and Shyh-Chang N. Oocyte Factors Suppress Mitochondrial Polynucleotide Phosphorylase to Remodel the Metabolome and Enhance Reprogramming. Cell Rep 2015; 12(7):1080-8. [PMID: 26257174]
- Ho CZ, Deng L, Picone R, Abderazzaq F, Flanagan N, Chua DZ, Low BC, and Wu SK. The Senescence-Associated Secretory Phenotype constitutes HIF-1α activation but is independent of micronuclei-induced cGas/Sting activation. Mol Biol Cell 2025;:mbcE24100445. [PMID: 41296514]
- Ho CZ, Lawther DB, Huang CB, Bharathkumar S, Beh SW, Poon EW, Lim PS, Lee KW, Felisha C, Tay SC, Yow I, Young JL, Wu SK, and Low BC. Protocol for AI-assisted quantitative analysis and setup of tumor spheroid invasion into tissue. STAR Protoc 2025; 6(4):104140. [PMID: 41075251]
- Sanz-Moreno V, and Low BC. Cell signaling across scales in health and disease. Curr Opin Cell Biol 2025; 97:102581. [PMID: 40946374]
- Xiao J, Ang JW, Zhong X, Wong DCP, T T, Yow I, Lee CJM, Foo RS, Kanchanawong P, and Low BC. Coordination of Focal Adhesion Nanoarchitecture and Dynamics in Mechanosensing for Cardiomyoblast Differentiation. ACS Appl Mater Interfaces 2025;. [PMID: 39778877]
- Shankar S, Liu Y, Tulsian NK, Low BC, Lin Q, and Sivaraman J. Insights into the regulation of CHIP E3 ligase-mediated ubiquitination of neuronal protein BNIP-H. PNAS Nexus 2024; 3(12):pgae536. [PMID: 39703232]
- Liu OX, Lin LB, Bunk S, Chew T, Wu SK, Motegi F, and Low BC. A ZO-2 scaffolding mechanism regulates the Hippo signalling pathway. FEBS J 2024;. [PMID: 39462647]
- Aureille J, Prabhu SS, Barnett SF, Farrugia AJ, Arnal I, Lafanechère L, Low BC, Kanchanawong P, Mogilner A, and Bershadsky AD. Focal adhesions are controlled by microtubules through local contractility regulation. EMBO J 2024;. [PMID: 38769437]
- Lee CJM, Autio MI, Zheng WH, Song Y, Wang SC, Wong DCP, Xiao J, Zhu Y, Yusoff P, Yei X, Chock WK, Low BC, Sudol M, and Foo RS. Genome-Wide CRISPR Screen Identifies an NF2-Adherens Junction Mechanistic Dependency for Cardiac Lineage. Circulation 2024;. [PMID: 38752370]
- Wu Y, Cheng J, Qi J, Hang C, Dong R, Low BC, Yu H, and Jiang X. Three-dimensional liquid metal-based neuro-interfaces for human hippocampal organoids. Nat Commun 2024; 15(1):4047. [PMID: 38744873]
- Shankar S, Chew TW, Chichili VPR, Low BC, and Sivaraman J. Structural basis for the distinct roles of non-conserved Pro116 and conserved Tyr124 of BCH domain of yeast p50RhoGAP. Cell Mol Life Sci 2024; 81(1):216. [PMID: 38740643]
- Tijore A, Margadant F, Dwivedi N, Morgan L, Yao M, Hariharan A, Chew CAZ, Powell S, Bonney GK, and Sheetz M. Ultrasound-mediated mechanical forces activate selective tumor cell apoptosis. Bioeng Transl Med 2024; 10(2):e10737. [PMID: 40060768]
- Jain K, Kishan K, Minhaj RF, Kanchanawong P, Sheetz MP, and Changede R. Immobile Integrin Signaling Transit and Relay Nodes Organize Mechanosignaling through Force-Dependent Phosphorylation in Focal Adhesions. ACS Nano 2025;. [PMID: 39760672]
- Mierke CT, Hu X, Yao M, Schink KO, and Sheetz M. Editorial: Tumor cell mechanosensitivity: molecular basis. Front Cell Dev Biol 2024; 12:1484725. [PMID: 39310224]
- Fu C, Dilasser F, Lin S, Karnat M, Arora A, Rajendiran H, Ong HT, Mui Hoon Brenda N, Phow SW, Hirashima T, Sheetz M, Rupprecht J, Tlili S, and Viasnoff V. Regulation of intercellular viscosity by E-cadherin-dependent phosphorylation of EGFR in collective cell migration. Proc Natl Acad Sci U S A 2024; 121(37):e2405560121. [PMID: 39231206]
- Hu X, Jalal S, Yao M, Bakke O, Margadant F, and Sheetz M. Differential Talin cleavage in transformed and non-transformed cells and its consequences. Front Cell Dev Biol 2024; 12:1430728. [PMID: 39086658]
- Lin S, Changede R, Farrugia AJ, Bershadsky AD, Sheetz MP, Prost J, and Rupprecht J. Membrane Tilt Drives Phase Separation of Adhesion Receptors. Phys Rev Lett 2024; 132(18):188402. [PMID: 38759206]
- Jain K, Minhaj RF, Kanchanawong P, Sheetz MP, and Changede R. Nano-clusters of ligand-activated integrins organize immobile, signalling active, nano-clusters of phosphorylated FAK required for mechanosignaling in focal adhesions. bioRxiv 2024;. [PMID: 38464288]
- Jain K, Pandey A, Wang H, Chung T, Nemati A, Kanchanawong P, Sheetz MP, Cai H, and Changede R. TiO2 Nano-Biopatterning Reveals Optimal Ligand Presentation for Cell-Matrix Adhesion Formation. Adv Mater 2024;:e2309284. [PMID: 38340044]
- Jain K, Lim KYE, Sheetz MP, Kanchanawong P, and Changede R. Intrinsic self-organization of integrin nanoclusters within focal adhesions is required for cellular mechanotransduction. bioRxiv 2023;. [PMID: 38045378]
- Oh D, Liu X, Sheetz MP, and Kenney LJ. Small, Dynamic Clusters of Tir-Intimin Seed Actin Polymerization. Small 2023;:e2302580. [PMID: 37649226]
- Mitra A, Cutiongco MFA, Burla R, Zeng Y, Na Q, Kong M, Vinod B, Nai MH, Hübner B, Ludwig A, Lim CT, Shivashankar GV, Saggio I, and Zhao W. Acute chromatin decompaction stiffens the nucleus as revealed by nanopillar-induced nuclear deformation in cells. Proc Natl Acad Sci U S A 2025; 122(19):e2416659122. [PMID: 40343993]
- Das R, Sakaue T, Shivashankar GV, Prost J, and Hiraiwa T. Chromatin Remodeling Due to Transient-Link-and-Pass Activity Enhances Subnuclear Dynamics. Phys Rev Lett 2024; 132(5):058401. [PMID: 38364140]
- Jiang K, Lim SB, Xiao J, Jokhun DS, Shang M, Song X, Zhang P, Liang L, Low BC, Shivashankar GV, and Lim CT. Deleterious Mechanical Deformation Selects Mechanoresilient Cancer Cells with Enhanced Proliferation and Chemoresistance. Adv Sci (Weinh) 2023;:e2201663. [PMID: 37218524]
- Das R, Sakaue T, Shivashankar GV, Prost J, and Hiraiwa T. How enzymatic activity is involved in chromatin organization. Elife 2022; 11. [PMID: 36472500]
- Yuan L, Roy B, Ratna P, Uhler C, and Shivashankar GV. Lateral confined growth of cells activates Lef1 dependent pathways to regulate cell-state transitions. Sci Rep 2022; 12(1):17318. [PMID: 36243826]
- Venkatachalapathy S, Sreekumar D, Ratna P, and Shivashankar GV. Actomyosin contractility as a mechanical checkpoint for cell state transitions. Sci Rep 2022; 12(1):16063. [PMID: 36163393]
- Venkatachalapathy S, Jokhun DS, Andhari M, and Shivashankar GV. Single cell imaging-based chromatin biomarkers for tumor progression. Sci Rep 2021; 11(1):23041. [PMID: 34845273]
- Ghanbarzadeh Nodehi S, Shivashankar GV, Prost J, and Mohammad-Rafiee F. The characteristics of nuclear membrane fluctuations in stem cells. J R Soc Interface 2021; 18(176):20201010. [PMID: 33715401]
- Yang KD, Belyaeva A, Venkatachalapathy S, Damodaran K, Katcoff A, Radhakrishnan A, Shivashankar GV, and Uhler C. Multi-domain translation between single-cell imaging and sequencing data using autoencoders. Nat Commun 2021; 12(1):31. [PMID: 33397893]
- Lee Y, and Shivashankar GV. Analysis of transcriptional modules during human fibroblast ageing. Sci Rep 2020; 10(1):19086. [PMID: 33154459]
- Fokin AI, Lin Y, Guschin DY, Chen H, James J, Yan J, Silberzan P, and Gautreau AM. Identification of PKN2 and MOB4 as Coordinators of Collective Cell Migration. Adv Sci (Weinh) 2025;:e02907. [PMID: 41276909]
- Liu X, Liu J, Wang Y, Yao M, Baker KB, Klapholz B, Brown NH, Goult BT, and Yan J. The mechanical response of vinculin. Sci Adv 2025; 11(44):eady6949. [PMID: 41171905]
- Yeow J, Chia CG, Lim NZ, Zhao X, Yan J, and Chng S. Structural Insights into the Force-Transducing Mechanism of a Motor-Stator Complex Important for Bacterial Outer Membrane Lipid Homeostasis. J Am Chem Soc 2025;. [PMID: 40589080]
- Liu J, Deng Y, and Yan J. Decoding force-transmission linkages for therapeutic targeting and engineering. APL Bioeng 2025; 9(2):021504. [PMID: 40520648]
- Wang Y, Zhang Y, Yu M, Xiu P, Jia Y, Chen H, Le S, Qian J, and Yan J. Salt-bridge mediated cooperativity and mechanical stabilization of tandem spectrin repeats. Nanoscale Horiz 2025;. [PMID: 40492359]
- Wang J, Fu C, Chang S, Stephens C, Li H, Wang D, Fu YC, Green KJ, Yan J, and Yi R. PIEZO1-mediated calcium signaling reinforces mechanical properties of hair follicle stem cells to promote quiescence. Sci Adv 2025; 11(22):eadt2771. [PMID: 40435254]
- Hui W, Lei K, Liu Y, Huang X, Zhong Y, Chen X, Wei M, Yan J, Shen R, Mak P, Martins RP, Yi S, Wang P, and Jia Y. Identification and Drug Screening of Single Cells from Human Tumors on Semiconductor Chip for Cancer Precision Medicine. Adv Sci (Weinh) 2025;:e2503131. [PMID: 40271835]
- Maupérin M, Sun Y, Glandorf T, Oswald TA, Klatt N, Geil B, Mutero-Maeda A, Méan I, Jond L, Janshoff A, Yan J, and Citi S. A feedback circuitry involving γ-actin, β-actin and nonmuscle myosin-2 A controls tight junction and apical cortex mechanics. Nat Commun 2025; 16(1):2514. [PMID: 40082413]
- Huang W, Lim Z, and Yan J. Ions shaping the mechanics of chromosomes in mitosis. Nat Mater 2024;. [PMID: 39472751]
- Le S, Yu M, Fu C, Heier JA, Martin S, Hardin J, and Yan J. Single-molecule force spectroscopy reveals intra- and intermolecular interactions of Caenorhabditis elegans HMP-1 during mechanotransduction. Proc Natl Acad Sci U S A 2024; 121(37):e2400654121. [PMID: 39236238]