The Crown JeWell of organoid imaging
An interdisciplinary team from the Mechanobiology Institute, National University of Singapore combined imaging, microfabrication, and biology to develop JeWells – an innovative platform for growing and imaging organoids in 3D. This study was published in Nature Methods and featured on the July 2022 cover, https://www.nature.com/articles/s41592-022-01508-0
Organoids are lab-grown 3D cells or tissues aggregates that mimic organs. As in vitro models of the original organs, they enable scientists to modify and observe organs outside of the body. This helps scientists to explore how our anatomy develops, how disease spreads in our body, and if a drug works or causes side effects. For example, heart ‘injury’ organoids are used to study the regenerative potential of heart muscle cells after they receive damage.
Despite this potential, there are many challenges involved in growing and observing organoids. For accurate information organoids need to be consistent and reproducible. Unfortunately, the 3D nature of organoid development can result in biophysical variability which can skew the accuracy of the observations. Moreover, current methods for growing organoids are not suited for the efficient 3D observation of large numbers of organoids. The major challenge to harnessing the potential of organoids in health and disease requires a parallel approach that enables growth of large numbers – hundreds or thousands – of organoids, coupled with the ability for live observation of the development of all these organoids for characterization.
Developing a multidisciplinary approach for organoid growth and analysis
To tackle this challenge, the labs of Assoc. Prof. Virgile Viasnoff, Research Asst. Prof. Anne Beghin and Research Asst. Prof. Gianluca Grenci of the Mechanobiology Institute set out to create a one-stop automated platform for growing, imaging, and characterising organoids. The result was achieved by synergistically combining advanced microfabrication, imaging, and biological protocols, to develop an original approach capable of growing reproducible organoids over a long-term time period while allowing for live and 3D imaging of these organoids that can be done at high-speed and scaled up for high-content analysis powered by artificial intelligence.
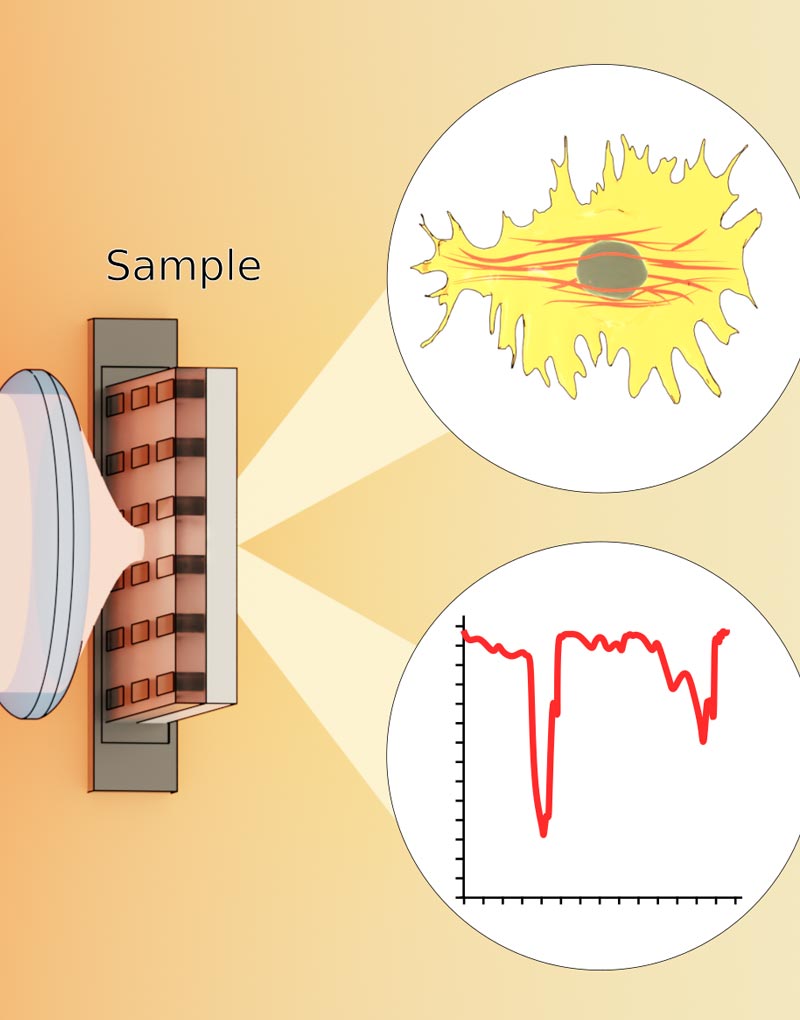
At the core of the invention are microfabricated chips known as the “JeWell chip”. Each chip can contain up to thousands of JeWells, which are microscopic, pyramid-shaped chambers (Link to Figure). At the top of these pyramids researchers can introduce single cells, or a collection of cells required for organoid formation, which will sit at the bottom of the chamber and mature into 3D organoids. Since organoids of different kind come in a variety of unique physical and growth requirements, the shape, size, and condition of JeWells can be customised to cater to them accordingly. For example, to grow the vessels that transport bile from the liver, JeWells of elongated shape can be made to fit the extension of tubular organoids.
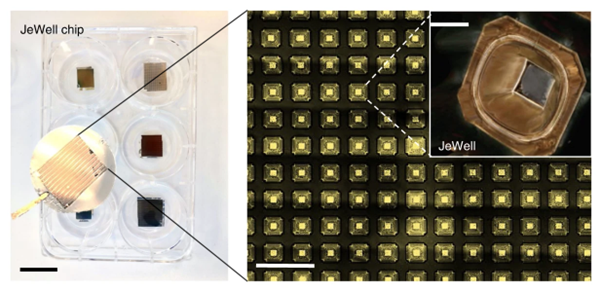
Photo of JeWell chip
Each wall of the pyramidal cavities in the JeWell is fabricated to reflect light, effectively acting as a mirror. These micro-mirrors work together with a light source introduced through the same single lens used for acquiring images of the samples, a method known as “single-objective selective-plane illumination microscopy (soSPIM)”.
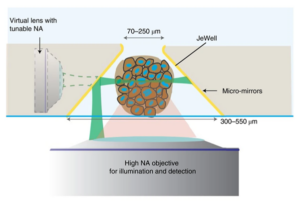
Principle of soSPIM imaging
Briefly, the soSPIM working principle uses the micro-mirror (inclined at exactly 45° compared to the horizontal base of the JeWell) to reflect a narrow light beam shone from below at a 90° angle towards the sample, in this case an organoid. The narrow beam cuts across the sample horizontally, illuminating only a cross-section or a single “plane” of it. The light intersecting the plane is then picked up by the detector, and by scanning the beam at different height on the micro-mirrors, a 3D scan of the sample within the JeWells is reconstructed layer by layer. The original soSPIM technology was developed in collaboration with the CNRS group of Jean Baptiste Sibarita and Remi Galland at the IINS (Bordeaux, FRANCE).
A feature that the soSPIM shares with other light-sheet imaging approaches is that is minimizes light exposure to the biological sample. Prolonged exposure to light, especially the laser light used for in-depth imaging can cause damage to living organoid cultures. The light-sheet scheme utilized in soSPIM limits light exposure to only the cross-section of the 3D organoid that is being imaged and also facilitates fast image capture. The JeWell surfaces also have an antifouling coating to prevent unwanted microorganism growth. Together, these features allow long-term growth and microscopic monitoring of live 3D organoids. The research team were able to grow 3D organoids of nervous system tissue, intestine, liver, and cancer tumours for up to several weeks. Each of these organoids developed characteristic mechanobiological features of the human organ, such as bile duct formation in liver organoids.
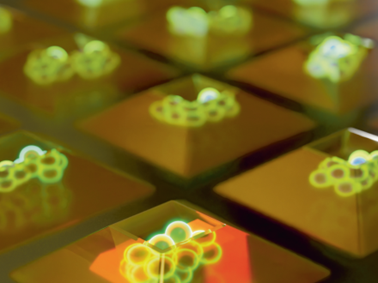
Illustration of cell aggregates growing in JeWells
Another advantage of JeWells is the ease with which they can be imaged. Using a standard inverted microscope, up to 300 organoids per hour can be imaged. The organoids can be visualised at multiple scales – at the multicellular organoid level, down to individual cells and even subcellular imaging of individual cell components. This unlocks the potential for JeWells to be used for organoid screening. The ability to capture images of subcellular components at high detail can be augmented using image analysis software powered by AI systems to monitor the status of different cell components or metabolic events such as cell division.
The research team were able to successfully detect all the nuclei in an organoid, classify dividing cells according to their stage of proliferation, identify dividing or dead cells, recognise differentiating cells, and classify cells based on their shape with high accuracy. This means that hundred or organoids can be classified automatically using AI, saving the labour and time needed for manual classification.
This publication demonstrates how the JeWell platform is a powerful and comprehensive all-in-one platform for culturing, 3D-imaging and quantifying organoids. One of the prospective applications for this technology is high-content screening using human organoids, which could possibly supersede the need for animal testing. The research team are busy upgrading the technical aspects of JeWells, by testing and building up a library of available JeWell geometries to cater to larger or more complex organoids. One other exciting side-project of JeWells involves the space agency NASA, who are interested in launching JeWells into space for observing organoid growth in zero-gravity environments.