soSPIM Developed at MBI
Pui Yee Loh | MAY 2015
Recent advances in microscopy have allowed scientists to image biological samples in 3D for extended periods of time, without causing damage to the sample. Combined with super-resolution imaging, this means that the activity of single proteins can be followed within individual cells or tissues, providing new insight into protein function, and importantly, how protein dysregulation can lead to disease. Unfortunately, these imaging techniques are still prohibitively complicated and expensive for most labs. Galland et al., 3D high- and super-resolution imaging using single-objective SPIM. Nature Methods, 11 May 2015, doi:10.1038/nmeth.3402.
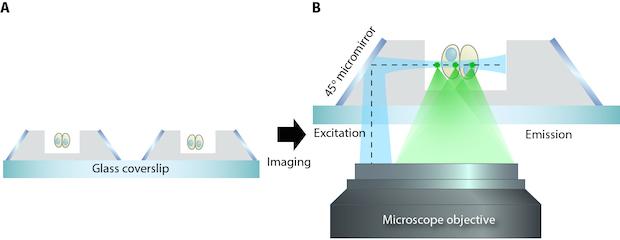
Schematic showing the soSPIM technique. A) Cells can be grown in the microfabricated wells, holding them in place for imaging. B) The 45o micromirrors reflect the excitation beam (dotted line) from a single objective, through the sample, and the resultant emitted fluorescence signal is captured by the same objective.
Microfabrication leads to a new microscopy method
Despite recent progress in the development of super-resolution microscopy only a few techniques, such as total internal reflection fluorescence (TIRF) illumination and interferometric photoactivated localization microscopy (iPALM), enable single- molecule imaging. These techniques are limited to capturing 2D images near the surface of the glass coverslip, or 3D images within the first micrometre (μm) of the coverslip. A typical human skin cell is 30 μm in height.
Selective Plane Illumination Microscopy (SPIM) is a technique that enables 3D super-resolution imaging of thicker samples at a single-cell level. This technique selectively illuminates a single plane of the specimen by directing a focused light sheet from one side while capturing the fluorescence signal through a second objective positioned perpendicularly to the light sheet. A 3D image is reconstructed from collected images of individual cell sections. However, this approach requires a complicated 2-objective system and special sample holder which makes it incompatible with standard microscope systems.
The need for super-resolution 3D imaging
With the aim of providing a simple, yet versatile microscopy technique that can identify single proteins anywhere within a cell, and allow cellular organization to be assessed in 3D, researchers at the Mechanobiology Institute (MBI), National University of Singapore, have developed an improved SPIM technique that requires only a single objective, called the soSPIM.
This new technique provides researchers the ability to monitor the activity of single proteins on their existing microscope systems.
This technique, developed by Assoc Prof Virgile Viasnoff, Principal Investigator at MBI, and CNRS, France, utilizes an array of micromirrored wells that are produced via microfabrication. Each mirror, which is inclined at precisely 45°, serves as both a means to direct the excitation beam, and also to hold the sample. Together with a beam steering add-on unit, these micromirrors allow for both the excitation beam, and fluorescence signal, that is viewed through the microscope, to pass through a single standard, objective lens. The soSPIM technique exhibited fast response and good sectioning capability for 3D imaging of a whole cell up to 30 μm above the coverslip. It was also able to identify single proteins, deep within the cell.
With the microfabricated mirror and sample holder being produced independently of the microscope system, this technique is compatible with standard inverted microscopes and high numerical aperture immersion objective lenses. This will provide more researchers the ability to monitor the activity of single proteins on their existing microscope systems.
Protein dysregulation may result in changes to any part or process within the cell. Being able to see these changes is crucial for researchers to fully understand why certain disease states arise. This means being able to visually observe the whole cell in all three dimensions as well as at the single-molecule level. The development of the soSPIM technique not only makes these needs attainable, but does so using conventional inverted microscope technology.