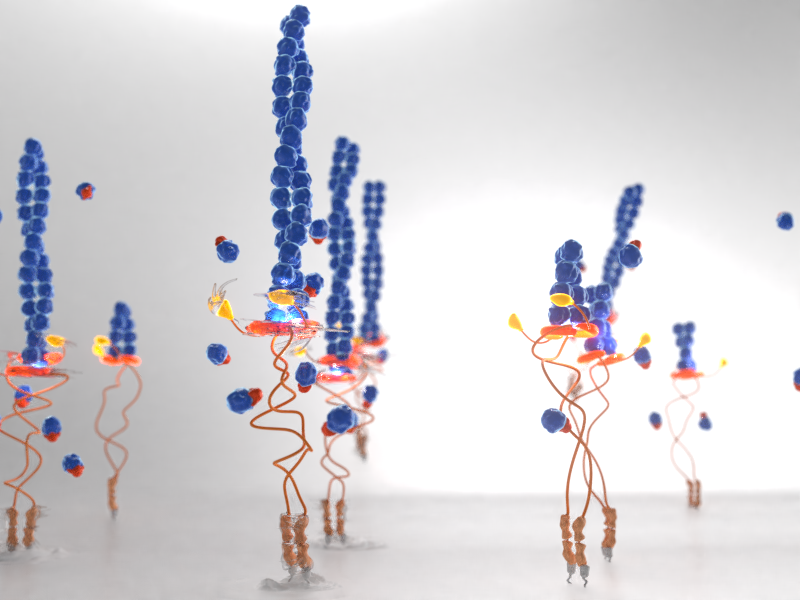
Sensing Force and Torque
Mechanical regulation of actin polymerization by formins
Edited by Andrew MS Wong, PhD | 23 NOV 2017 | Illustration by Diego Pitta de Araujo
A team led by Mechanobiology Institute (MBI) Principal Investigators Prof. Jie Yan and Prof. Alexander Bershadsky have revealed how formin family proteins sense pulling force and torque during formin mediated actin polymerization. This study was published in Nature Communications. Yu et al., mDia1 senses both force and torque during F-actin filament polymerization. Nature Communications, 2017 Nov; 8:1650, doi:10.1038/s41467-017-01745-4.
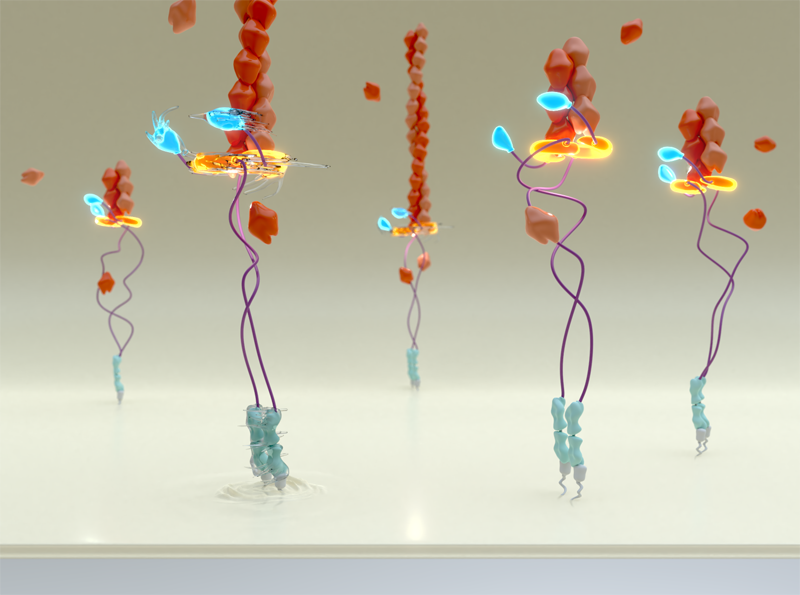
Formin homodimers assemble as a ring (gold) at the barbed end of the actin filament (red). When locked in place (right), this ring prevents addition of actin monomers to the barbed end and inhibits actin polymerization. However, the application of force changes the conformation of the formin ring so that it can rotate around the actin filament (left), speeding up actin polymerization nearly 10-fold and resulting in actin filament elongation.
Forcing apart the dual role of formins
In the body, cells alter their shape and behavior in response to internal and external mechanical forces by remodeling of the actin and microtubule cytoskeletons, the dynamic structural scaffolding proteins that support the cell. This remodeling is regulated by numerous force sensing proteins, such as members of the formin family, which are key molecular regulators of cytoskeletal assembly and organization.
Understanding how formin can sense force and torque, and use this information to regulate actin polymerization during cellular deformation could reveal the role of formin in maintaining cytoskeletal integrity.
All formin family proteins have FH1 and FH2 domains, and assemble as homodimers (i.e. two individual formins bind to each other). The dimerized FH2 domains assemble into a ring-like structure, which encircles and binds to the barbed end of an actin filament where polymerization, the elongation of the filament by addition of actin monomers, takes place.
Different formin domains promote or inhibit actin polymerization
On one hand, the actin associated formin promotes actin polymerization by increasing the local concentration of actin monomers through an interaction between the FH1 domains of formin and the actin monomer binding protein profilin. On the other hand, as the FH2 domain is bound on the barbed end of actin, formin itself acts as a gate against recruitment of new actin monomers to the actin filament, imposing a significant barrier that suppresses actin polymerization. Therefore, formin has a dual identity, with the FH1 domain promoting actin polymerization, yet the FH2 ring inhibits actin polymerization. Understanding how these opposing roles of formin balance each other to enable actin polymerization has been a question that scientists have long sought to answer.
An important hypothesis of formin-mediated actin polymerization is that mechanical force applied to a formin dimer attached to an actin filament may cause the ‘closed’ ring of the FH2 domains to open up, thereby speeding up actin polymerization. In spite of its simplicity, it has been technically challenging to test this hypothesis in experiments.
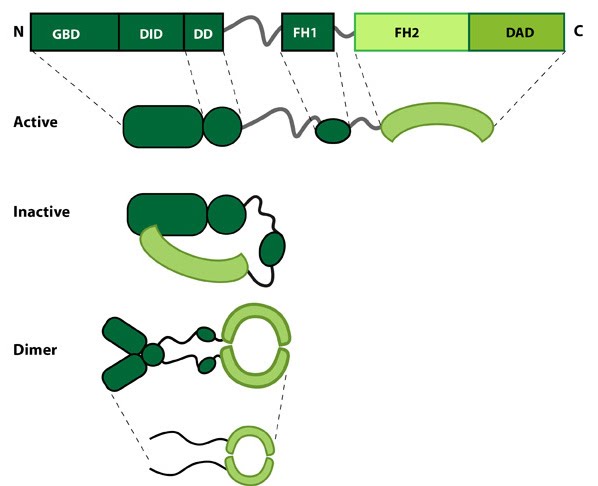
This schematic diagram illustrates the molecular organization of formin. The FH1 domain helps recruit actin monomers. The FH2 domain assembles into a ring-shaped complex in dimerized formin, and binds to the actin filament. Image adapted from https://www.mechanobio.info/figure/1384240860778/.
Unlocking formin-mediated actin polymerization with force and torque
By developing a sophisticated single-molecule manipulation technology, MBI research fellow Miao Yu and colleagues were able to successfully observe and measure formin-dependent actin polymerization under different mechanical constraints applied to actin and formin. The team found that, when the relative rotation between an actin filament and the formin attached to the barbed end of the filament is not constrained, a force of a few picoNewtons can increase the actin polymerization speed by almost 10 times compared to the actin polymerization speed in the absence of force. In contrast, when the relative rotation between an actin filament and the formin was inhibited, this force-dependence of actin polymerization disappears. Therefore, the study not only supports the previously proposed hypothesis on force-dependent actin polymerization, but also reveals a novel torque-sensing function of formin. This torque-sensing function may explain the dual role of formin in both promoting and inhibiting actin polymerization.
This study revealed that formin is able to sense and respond to both force and torque, and this mechanosensing ability is what enables it to mediate actin polymerization. As the actin cytoskeleton is subject to complex mechanical constraints in living cells, these results provide important insights into how formin senses these mechanical constraints and regulates actin organization accordingly. Understanding how formin can sense tension changes and regulate actin polymerization during cellular deformation could reveal the role of formin in maintaining cytoskeletal integrity.