HER2 can HEAR2
Steven Wolf, PhD and Sruthi Jagannathan, MSc. Illustrations by Diego Pitta de Araujo, PhD | May 2017
An international team of researchers from the Mechanobiology Institute, National University of Singapore and Columbia University, USA have identifed the role of ERBB family receptors, including EGFR and HER2 in integrating mechanical and biochemical signals that are essential for promoting cellular behavior such as its growth and movement. This study is published in Nature Materials [doi: 10.1038/nmat4893].
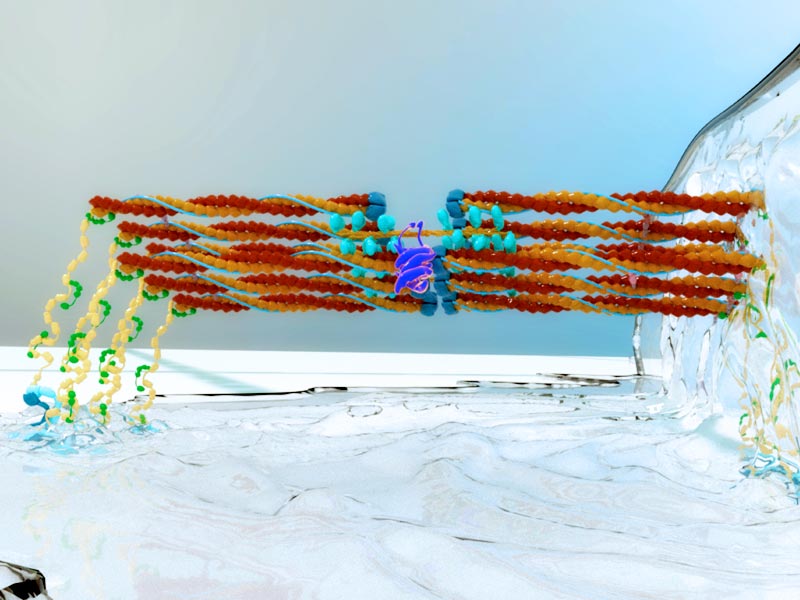
An illustration of the activation of actomyosin contractions by HER2 (in purple) in response to the stiffness of the extracellular surface.
How the ‘deaf’ receptor responds to mechanical signals
In recent studies from the Mechanobiology Institute, National University of Singapore, the mechanisms cells use to measure the physical properties of their surroundings were described for the first time. The studies, which were conducted by researchers from the laboratory of Prof Michael Sheetz, revealed subcellular contractile machines that pinch on the material surrounding cells as a means of determining how soft or stiff the environment is. This process is now widely known as mechanosensing, and evidence is mounting to suggest that the information gathered can define cellular growth patterns, initiate cell death programs, and even instruct cells on the need to relocate to other regions in the tissue.
Of course, our bodies, and the tissues in which individual cells reside, are highly dynamic, and physical or mechanical cues, are just one factor that determine cell behavior. Both inside and outside of cells, the environment is teeming with biochemical molecules; from cytokines to hormones, and even small chemical molecules such as carbon monoxide or hydrogen sulfide. Not surprisingly, the process of mechanosignaling is intertwined with biochemical signaling.
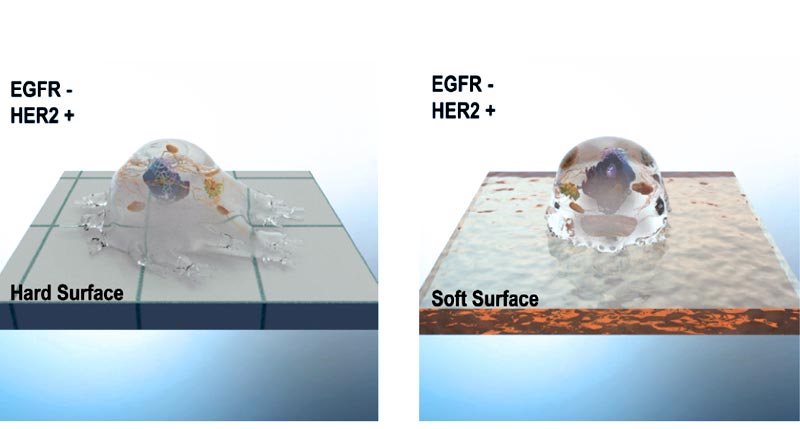
HER2 activates mechanosensing on rigid surfaces even in the absence of other ERBB family members such as EGFR. While HER2 enhances actomyosin- dependent contractions and cell spreading on hard surfaces, it did not have any effect on cellular contractility and spreading on soft surfaces.
Despite having described key machines within the cell that facilitate mechanosensing, the researchers had yet to crack one key question; understanding what was controlling the contractile pinching machines. To answer this question MBI Research Assistant Yang Bo Beverly from Prof Sheetz’ lab, turned her attention to exploring the influence of biochemical signaling on the activation of the contractile mechanosensors.
HER2 activates mechanosensing on rigid matrices
This study makes clear that the ability of cells to sense and respond to their physical environment must be considered in the development of future cancer drugs targeting biochemical receptors.
Of particular interest was the role of a protein known as HER2, as its levels are often abnormally increased in cancer cells, and cancer cells often exhibit altered growth patterns, disrupted cell death programmes and increased motility.
The HER2 protein belongs to a larger family of specialized receptor proteins known as the ERBB family. HER2 was known to detect signals from outside the cell, and convey these signals to the nucleus. However, HER2 was also unique from other members of the ERBB family, as it does not bind to any known signaling molecule and can only pass on biochemical information when in the presence of another ERBB family member. It had become known by scientists as a ‘deaf’ receptor.
However, as Yang and colleagues revealed, HER2 is indeed responsible for the activation of the pinching machines, even when other ERBB family members were absent. More importantly, activation by HER2 only induced rigidity sensing contractions when the cells were on hard surfaces, and not on softer tissues. This suggested that HER2 was not ‘deaf’ after all, but rather, it ‘hears’ a different type of signal. HER2’s ability to detect mechanical signals was independent of other ERBB receptors, and allowed the cell to respond by increasing contractions, to subsequently promote cell growth and movement.

MBI Research Assistant Yang Bo Beverly from Prof Sheetz’ lab.
It remains possible that the increased levels of HER2 in cancers contribute to the altered response of cancer cells to mechanical signals. However, what is more important, is the complex relationship between mechanical and biochemical signaling, and how this relationship can be disrupted in cancer. Not only can mechanical signals drive biochemical pathways, but the biochemical pathways can in turn control the cellular mechanosensing machines. Any disruption to these controls can lead to a misinterpretation of the mechanical cell environment, and the erratic cell growth commonly associated with cancer.
Previously, the HER2 signaling pathway was targeted using single-drug therapeutic approaches, however these drugs failed to effectively prevent cancer growth. From the work presented in this study it becomes clear that the impact on the ability of cells to sense and respond to their physical environment must be considered in the development of future cancer drugs that target biochemical receptors.