From the Bottom Up
Andrew MS wong, phd | may 2017, illustration by diego pitta de araujo, phd
For more than a decade, the coordinated movement and rearrangement of cells during fruit fly embryonic development was thought to be controlled by contractile activity at the top of the cells, followed by the passive movement of the rest of the cell, including the base. However, scientists from the Mechanobiology Institute (MBI), National University of Singapore have discovered that protrusions extending at the bottom of the cell actually precede the movements at the top, and these basal protrusions play significant roles in directing tissue elongation. This study was published in Nature Cell Biology (Sun Z et al., Basolateral protrusion and apical contraction cooperatively drive Drosophila germ-band extension. Nature Cell Biology, 2017 Apr; 19(4):375-383. doi: 10.1038/ncb3497)
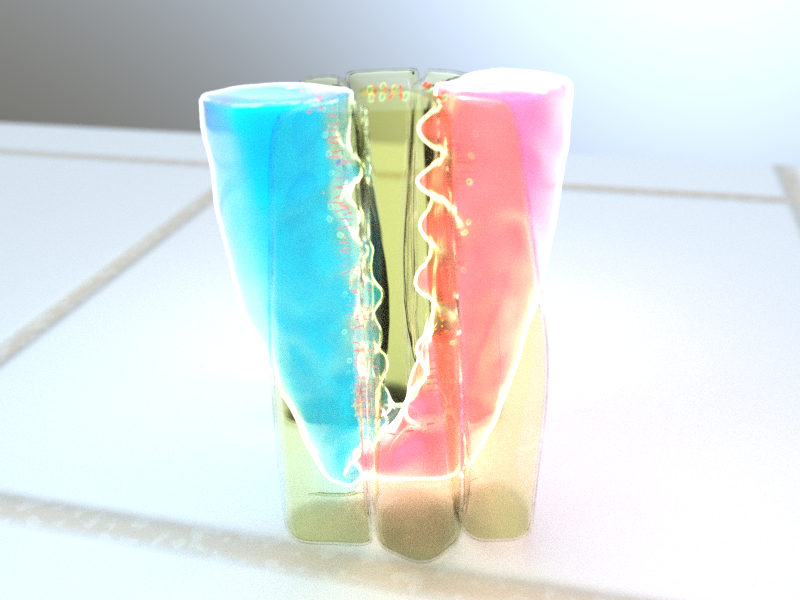
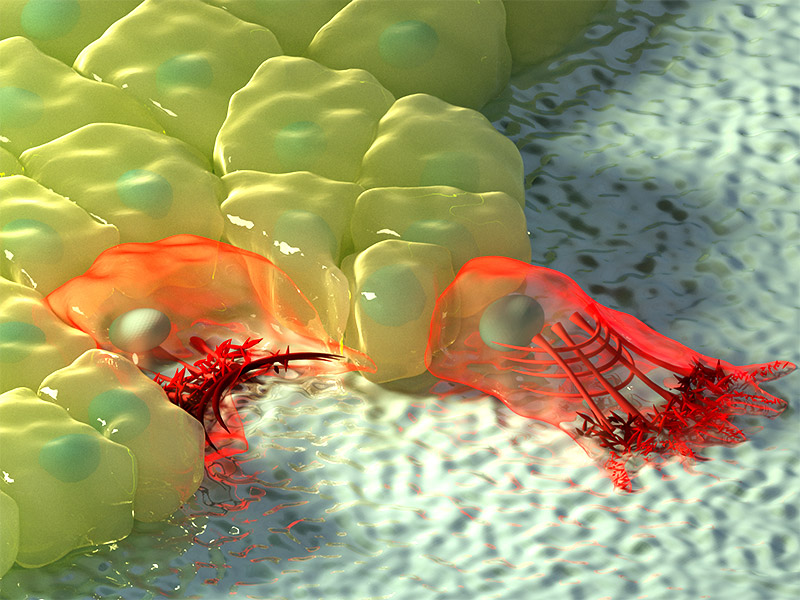
3D illustration of rosette formation. Protrusions extending from the bottom of dorsal (blue) and ventral (red) cells form a rosette at the base of the cell, while contractile activity between anterior/posterior cells (yellow) form a rosette at the top of the cell. Illustration by Diego Pitta de Araujo, PhD.
Uncovering the secret dynamics at the base of the cell
As embryos develop into an adult organism, they have to undergo numerous changes in shape and size. Sometimes this can be as a result of cell division and growth, but other times it is dependent on cellular rearrangement and coordinated collective cell movements.
One of the ways that cells undergo tissue elongation is by cell intercalation. This is the process by which neighbouring cells move and insert in between one another to rearrange their organization. This enables changes in the shape of the tissue, typically without a change in the overall number of cells. Often, cell intercalation leads to spreading of tissue along one axis, at the expense of narrowing along the perpendicular axis. An example of this occurs during fruit fly embryo development by a process known as germ-band extension. During this developmental stage, the germ-band tissue shrinks along the stomach-to-back axis, and extends to nearly double in length along the head-to-tail axis.
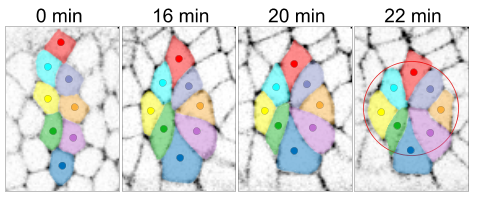
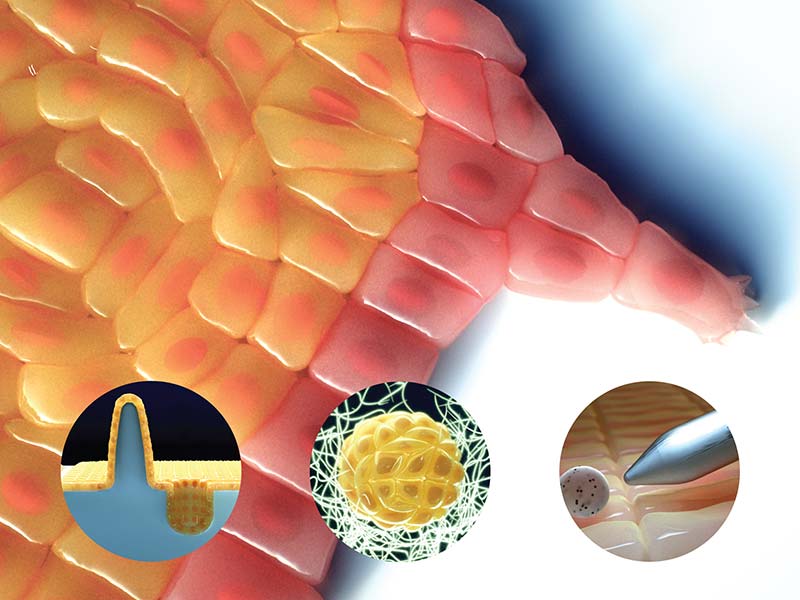
Apical rosette formation (red circle) during cell intercalation. Cells are colour-coded to illustrate movement and rearrangement. Adapted from Sun et al., doi: 10.1038/ncb3497
How does this cellular rearrangement take place? To visualize this, imagine looking down at a line of cells arranged vertically, from north-to-south. During germ-band extension, these cells need to rearrange horizontally along an east-to-west orientation. If this line was a queue of people, the simple solution would be for each person to leave the vertical line and rearrange themselves individually in a horizontal line. However, if this happened in a tissue it would require the cells to detach from one another, which would create gaps in the cell sheet and a damaging loss of tissue integrity. Cells remain connected to each other through cell adhesions, protein complexes located near the top of the cell. Fortunately, these cell adhesions are dynamic, so during cell intercalation the cells first rearrange into a flower-like ‘rosette’ pattern, leading to a decrease in the vertical axis, and from this rosette the cells then elongate in the horizontal axis
In the case of germ-band extension, years of scientific study has led to the general understanding that this dynamic remodeling of cell adhesions by contractile force is the key mechanism driving cell intercalation. The formation of an apical rosette near the top of the cell is a hallmark of this process. Surprisingly, given that cells are three-dimensional (3D), very little was known about cell dynamics at the bottom of the cell during rosette formation. This shortcoming was investigated by MBI Principal Investigator Asst. Prof. Yusuke Toyama, his graduate student Zijun Sun, and an interdisciplinary team of MBI scientists. Using advanced live imaging of germ-band extension in fruit fly embryos, coupled with 3D reconstruction, the research team were able to observe in detail the changes in shape from the top to the bottom that the cell undergoes during intercalation.
By observing the cell in 3D, this study has uncovered a new perspective on cell intercalations.
They discovered that a rosette pattern also formed at the base of the cell, and remarkably, these basal rosettes formed earlier than apical rosettes. Unlike apical rosettes, the formation of basal rosettes are not driven by contractile fibers, but instead by wedge-shaped protrusions that extended from the base of the cells, which then migrate towards each other during intercalation. Interfering with the formation of the basal rosettes leads to delayed germ-band extension, demonstrating that these basal rosettes are not merely a byproduct of the apical rosette, but are in fact active in cell intercalation. While basal and apical rosettes could form independently of one another, the cooperative activity of both rosettes is required for normal cell intercalation and germ-band extension.
By observing the cell in 3D, this study has uncovered a new perspective on cell intercalation. Previously, this process was thought to be solely directed by contractile activity at the top of the cell. However, this study turns our understanding of cell intercalation upside down, by clearly demonstrating that the first steps actually start at the base of the cell, where protrusive migration drives formation of basal rosettes. Understanding how cell intercalation depends on cellular movements ranging from the bottom to the top of the cell will provide fresh insight into embryonic development, and may help our knowledge of cancer, where the invasive, metastatic activity of tumour cells has many similarities with cell intercalation. Above all, this study shows how important it is to consider the other side of the coin – An unexpected fact might be waiting to be found…