Confined Migration Shapes the Bony Fate of Stem Cells
The findings broaden current understanding of mechanobiology by showing that confined migration itself acts as a physiological cue with lasting effects on cell identity.
Researchers from the Holle Lab at MBI reveal a new layer of control over stem cell differentiation, paving the way for new opportunities in regenerative medicine.
Moving through tight spaces is more than a logistical challenge for stem cells – it can fundamentally shape what they become. While it is well established that mechanical cues such as stiffness or shear stress can guide stem cell behaviour, less is known about how migration itself influences cell fate.
Researchers at the Mechanobiology Institute (MBI), National University of Singapore, have now shown that brief bouts of physical confinement during cell migration can bias the differentiation of human mesenchymal stem cells (hMSCs) and leave durable marks on the nucleus, ultimately guiding cells towards osteogenesis.
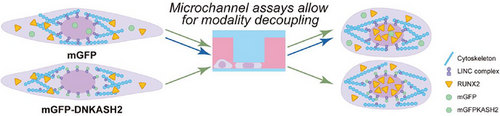
By exposing hMSCs to specific levels of physiological confinement, it is demonstrated that confinement can induce osteogenesis, and that this fate specification occurs due to nuclear deformation, not active force transduction across the nuclear envelope. Image from: https://doi-org.libproxy1.nus.edu.sg/10.1002/advs.202415407
In a study led by Xu Gao from the Holle Lab, published in Advanced Science, the team engineered polydimethylsiloxane (PDMS) microchannels to mimic the tight spaces within the extracellular matrix to study how human mesenchymal stem cells (hMSCs) respond to different degrees of confinement. It was observed that as cells forced their way through the narrower passages of 3 and 5 µm wide, they moved faster and underwent nuclear deformation which persisted long after the cells exited the channels. This mechanical memory translated to definitive shaping of cell identity.
At a molecular level, confined migration increased histone acetylation of H3K9, an epigenetic modification that relaxes chromatin structure and facilitiates gene expression. This was accompanied by greater nuclear localization of RUNX2, a master regulator of osteogeneis. In contrast, adipogenic signaling via PPARγ was suppressed, while chondrogenic marker SOX9 remained largely unaffected. The result suggests that confinement selectively guides lineage decisions.
The study also identified a central role for the mechanosensitive regulator YAP. A time-sensitive response after confined migration was discovered where YAP accumulated in the nucleus up till day 4 – suggesting that nuclear deformation is linked to transcriptional responses, but only at specific durations. This was further confirmed when YAP activity was inhibited, resulting in the prevention of confinement-induced activation of RUNX2, indicating that YAP activity is essential in translating the mechanical stress of migration into osteogenic commitment.
Importantly, these effects did not rely on the classical pathway of cytoskeletal force transmission through the LINC complex, which is often assumed to mediate mechanical sensing. Even when force transmission via the LINC complex was disrupted, confinement still drove osteogenic bias, suggesting that simple nuclear deformation was sufficient to drive confinement-sensitive stem cell differentiation.

Passive nuclear compression drives confinement-induced stem cell differentiation. Image from: https://doi-org.libproxy1.nus.edu.sg/10.1002/advs.202415407
The findings broaden current understanding of mechanobiology by showing that confined migration itself acts as a physiological cue with lasting effects on cell identity. They also point to new opportunities in regenerative medicine. For example, scaffolds and biomaterials could be designed to reproduce the degree of confinement found in tissues to improve the reliability of stem cell therapies, particularly those involving bone repair. As the effects of confinement persist after migration, there is also potential to mechanically precondition stem cells before transplantation, ensuring a more predictable therapeutic response.
By connecting migration through tight spaces to nuclear mechanics, epigenetic remodelling, and transcriptional regulation, the MBI team has revealed a new layer of control over stem cell differentiation. Their work underscores that cell fate is shaped not only by biochemical signals or substrate mechanics, but also by the physical constraints cells encounter as they move through tissues.