Cell polarity: An aurora over the pole
Written by Sruthi Jagannathan | Illustration provided by Motegi Lab | March 2019
Even before the fertilized egg or zygote can start dividing into daughter cells that form the future tissues and organs during the development of a multicellular organism, the symmetrical zygote needs to become asymmetrical or polarized in shape and molecular organization. The master switch that triggers the symmetry breaking process in the zygotes of the nematode worm, Caenorhabditis elegans, was identified in a recent study, led by MBI Principal Investigator Fumio Motegi. This work was published in Developmental Cell.
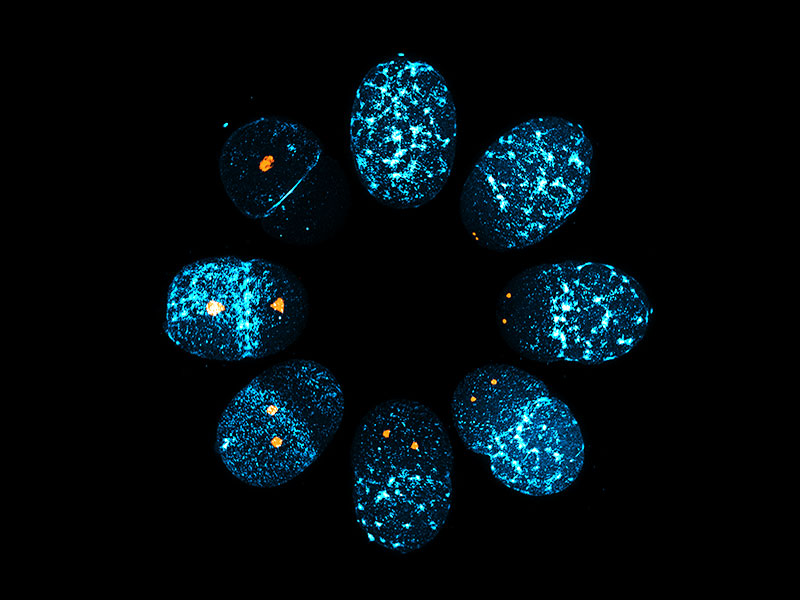
The image shows a C. elegans zygote undergoing symmetry breaking, during which spatial uniformity in cortical myosin-II (blue) is lost to establish an anterior-posterior polarity (clockwise order from the top). The local and global downregulation of cortical myosin-II networks during polarization is controlled by the mitotic kinase, Aurora-A (orange).
Aurora-A kinase is the symmetry breaking cue in C. elegans zygotes
Contrary to the simplified perception of cells as perfectly spherical bodies that is commonly depicted in school textbooks, the cells in our body assume asymmetrical or skewed physical shapes. This inherent asymmetry in their shape acts as an important spatial cue for many fundamental cellular processes, like how they divide or in which direction they move, to start and proceed normally.
“This study provides a key piece to the puzzle of how cell polarity is triggered and established. The single master switch, Aurora-A, creates ‘polar lights’ that cover one of the cell poles for symmetry breaking.”
–Dr Fumio Motegi
A wealth of information is now available on the structural and molecular level changes that take place when a cell assumes an asymmetrical or polarized state. Structurally, cells acquire top and bottom or front and rear surfaces, while at the molecular level, special protein groups called polarity regulators move to distinct regions in the cell cortex (a layer beneath the cell membrane). As a result, different cell regions acquire specific architectures and functions.
However, relatively little is known about the ‘master switch’ that initiates the spatial segregation of cellular components, in a process known as symmetry breaking. A central question that remains unanswered is the cue that guides the polarity regulators towards their destined locations. Asst. Prof. Fumio Motegi from the Department of Biological Sciences, National University of Singapore, and Principal Investigator at the Mechanobiology Institute (MBI) – a NRF Research Centre of Excellence that focuses on the mechanics of cellular and molecular processes – and the Temasek Life-Sciences Laboratory (TLL), set out to unravel the identity of this master switch.
By studying the process of symmetry breaking in zygotes from the nematode worm Caenorhabditis elegans, his team previously revealed how forces generated on the cortex by the contractions of the actin cytoskeleton (a filamentous framework made of actin and myosin proteins) could direct the movement of polarity regulators to their destined locations.
“This work nicely shows how important the measurement of dynamics is in developmental processes. Here we accomplished this with Fluorescence Correlation Spectroscopy, a versatile tool with single molecule sensitivity that allows to determine dynamics with good spatial resolution even in the complex environments of cells and organisms.”
–Dr Thorsten Wohland
Shortly after fertilization, actomyosin contractions in the cortex puts the surface of the zygote under tension. Upon symmetry breaking, spatially-controlled inhibition of actomyosin contractions lead to an imbalance in the surface tension, causing the cortical cytoskeletal networks to flow and transport the polarity regulators.
In this study, Dr. Motegi and his graduate student Peng Zhao ventured further to identify the cue that directly breaks symmetry in the cortical actomyosin contractions and leads to the initiation and establishment of polarity in C. elegans zygotes. After using a technique called RNA interference to block the synthesis of specific proteins involved in the polarization process, and observing the effects in live zygotes, they singled out a protein called Aurora-A (AIR-1 in C. elegans) as the master switch for symmetry breaking.
Aurora-A is a kinase (a type of protein that regulates the activity of other proteins by adding a phosphate molecule to them) that has well-known roles in controlling cell division by assembling centrosomes – a cellular organelle that organizes microtubule filaments and facilitates cell-cycle progression.
The researchers identified a two-stage process by which Aurora-A influences actomyosin contraction to initiate symmetry breaking and establish cell polarity. In the first stage, Aurora-A accumulates around centrosomes and suppresses actomyosin contractions locally in the surrounding cortex. This creates force differences along different regions of the cortex, and the resulting cortical flow transports myosins and other polarity proteins to the front of the zygote, thereby creating front-rear asymmetry.
In the second stage, Aurora-A diffuses into the cytoplasm and suppresses actomyosin contractions across the entire cortex. This prevents further cortex flows or movement of polarity regulators, effectively locking them in place.
“This work took advantage of the cross-departmental expertise in the use of microscopy-based tools at NUS. UV laser-induced ‘cellular surgery’ performed by us enabled manipulation of Aurora-A location in C. elegans zygotes to determine its influence on the symmetry breaking process.”
–Dr Yusuke Toyama
Intriguingly, the research team discovered that the role of Aurora-A in cell polarization was independent of its role in centrosome assembly and cell-cycle progression.
Through fruitful collaborations with colleagues at NUS, including Assoc. Prof. Yusuke Toyama (MBI) and Prof. Thorsten Wohland (Department of Biological Sciences), who brought their expertise in laser ablation and fluorescence correlation spectroscopy respectively, they demonstrated that the local accumulation of Aurora-A was sufficient to induce symmetry breaking via its kinase activity, regardless of the involvement of centrosomes.
Recent studies in the field have linked abnormal Aurora-A kinase activity with cancerous cell transformations. Will the findings from this study be able to shed some light on this pathogenic association?
Although there are still some missing links remaining before the whole cell polarization process is understood, the identification of Aurora-A as the master switch that controls cell polarization is a crucial step forward for uncovering further details about this essential biological process.
Importantly, growing evidence suggests that the process of polarization acts as a key checkpoint for preventing tumorigenic events in cells, and dysregulations in this process can ultimately lead to the onset of carcinogenesis. This study sets the stage for the development of novel therapeutic approaches for cancers based on manipulation and restoration of cell polarity.