Fumio MOTEGI
Assistant Professor, Mechanobiology Institute, National University of Singapore
fmotegi@tll.org.sg
Level 10 T-Lab
National University of Singapore
5A Engineering Drive 1
Singapore 117411
Research Program
Mechanotransduction in Tissues Group
Affiliations
Principal Investigator, Temasek Lifesciences Laboratory
Assistant Professor, Department of Biological Sciences, National University of Singapore
Cell polarity: Aurora over the pole
A recent study led by the Motegi Lab has identified the master switch that triggers the symmetry breaking process in the zygotes of the nematode worm, Caenorhabditis elegans. Learn more
Taking pole positions
How polarity proteins localize at opposite poles during symmetry breaking
Breaking cell symmetry
A force driven mechanism for establishing cell polarity
Fumio Motegi
Principal Investigator (2013-2021)
Research Areas
Mechanobiology of 1) cell polarity establishment and 2) soma-germline fate determination
Research Interests
Cell polarity, establishment of spatial asymmetry within a cell, is necessary for diverse processes in living organisms. Dedicated polarity proteins generate and maintain cellular asymmetry, leading to establishment of functional architecture in many types of cells. Despite the conserved role of polarity proteins, a fundamental question remains unanswered: How do developmental cues break cellular symmetry along the body axis during embryogenesis?
Movie 1: Breaking symmetry of embryonic polarity
Our group is interested in understanding the mechanics of 1) initiation of cell polarization and 2) spatial patterning of cellular asymmetry. A simple model system, C. elegans zygote, provides the unique opportunity to explore such initial procedures in cell polarization, as the zygotes do not rely on pre-localized proteins/RNAs or extrinsic cues to trigger asymmetry but instead undergo “de novo” polarization (Movie 1). By taking multi-disciplinary approach with genetics, biochemistry, and modern imaging technology, our group aims to:
- Define the nature of the cue that initiates polarization
- Assess the mechanics of spatial patterning of cellular asymmetry
- Understand the role of polarity kinases in germ-soma dichotomy
These views will delve into the basic principles of cell polarity and yield insights into how a developing embryo commits to somatic or germline cell fates with precision and accuracy. The molecular similarity of C. elegans to other systems makes it very likely that this project will highlight highly conserved mechanisms and lead to insights into asymmetric division in stem cells, prevention of cancer, and tissue regeneration.
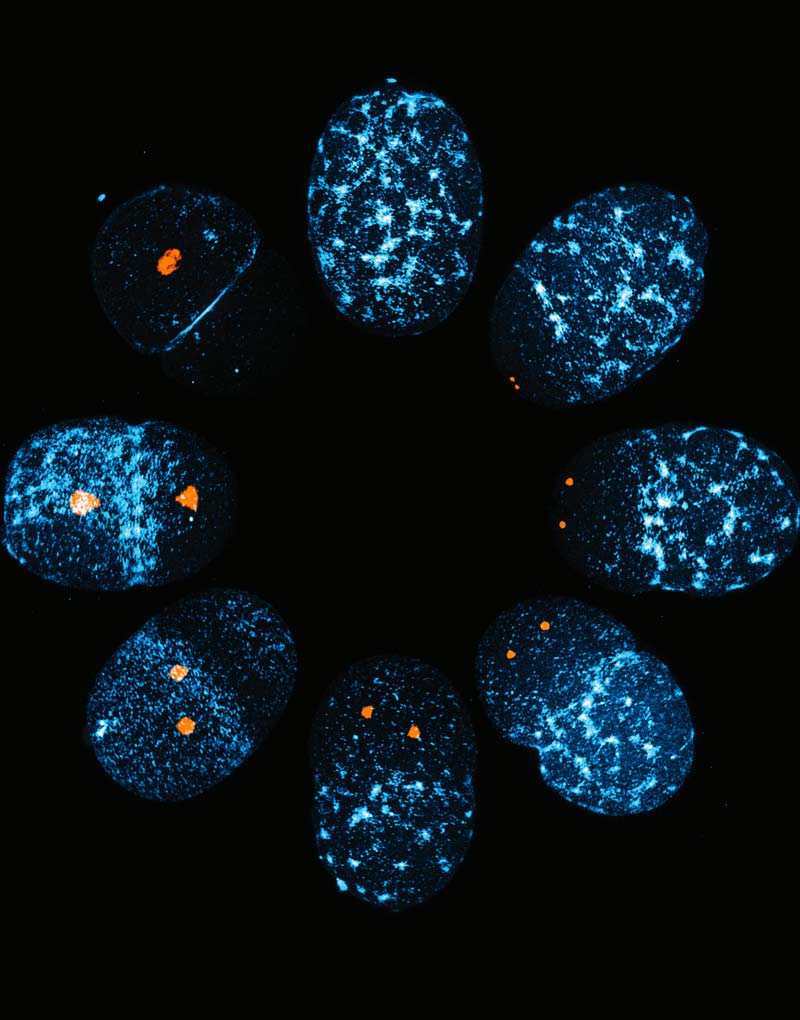
Symmetry breaking is the process by which cellular uniformity is broken to generate asymmetry in space. The cue that triggers symmetry breaking leads to the modulation of cell cortex to generate two distinct domains. The C. elegans zygote becomes polarized after fertilization by generating distinct domains: the anterior domain contains a complex of PAR-3, PAR-6, and atypical protein kinase C/aPKC (shown in magenta), and the posterior domain includes PAR-1 kinase and the RING protein PAR-2 (shown in green). Segregation of two sets of PAR proteins is critical for decision whether to become somatic cells or germ cells.
Biography
Fumio Motegi obtained his university diploma at Tokyo University of Science and completed his Master’s study and doctoral degree at the University of Tokyo. He did his postdoctoral work with Prof. Asako Sugimoto at RIKEN Center for Developmental Biology and with Prof. Geraldine Seydoux at Johns Hopkins University. He joined MBI and Temasek Lifesciences Laboratory as a Principal Investigator in August 2012 and holds a joint appointment as an Assistant Professor at Department of Biological Sciences at National University of Singapore.
Education
PhD University of Tokyo
Recent Publications
- Liu OX, Lin LB, Bunk S, Chew T, Wu SK, Motegi F, and Low BC. A ZO-2 scaffolding mechanism regulates the Hippo signalling pathway. FEBS J 2024;. [PMID: 39462647]
- Kimura K, and Motegi F. Fluid flow dynamics in cellular patterning. Semin Cell Dev Biol 2021;. [PMID: 34274213]
- Lim YW, Wen F, Shankar P, Shibata T, and Motegi F. A balance between antagonizing PAR proteins specifies the pattern of asymmetric and symmetric divisions in C. elegans embryogenesis. Cell Rep 2021; 36(1):109326. [PMID: 34233197]
- Gan WJ, and Motegi F. Mechanochemical Control of Symmetry Breaking in the Caenorhabditis elegans Zygote. Front Cell Dev Biol 2021; 8:619869. [PMID: 33537308]
- Motegi F, Plachta N, and Viasnoff V. Novel approaches to link apicobasal polarity to cell fate specification. Curr. Opin. Cell Biol. 2019; 62:78-85. [PMID: 31731147]
- Zhao P, Teng X, Tantirimudalige SN, Nishikawa M, Wohland T, Toyama Y, and Motegi F. Aurora-A Breaks Symmetry in Contractile Actomyosin Networks Independently of Its Role in Centrosome Maturation. Dev. Cell 2019; 48(5):631-645.e6. [PMID: 30861375]
- Ramanujam R, Han Z, Zhang Z, Kanchanawong P, and Motegi F. Establishment of the PAR-1 cortical gradient by the aPKC-PRBH circuit. Nat. Chem. Biol. 2018;. [PMID: 30177850]
- Zhang Z, Lim YW, Zhao P, Kanchanawong P, and Motegi F. ImaEdge: a platform for the quantitative analysis of cortical proteins spatiotemporal dynamics during cell polarization. J. Cell. Sci. 2017;. [PMID: 29113997]
- Wang S, Low TYF, Nishimura Y, Gole L, Yu W, and Motegi F. Cortical forces and CDC-42 control clustering of PAR proteins for Caenorhabditis elegans embryonic polarization. Nat. Cell Biol. 2017;. [PMID: 28737772]
- Ramanujam R, Low TYF, Lim YW, and Motegi F. Forceful patterning in mouse preimplantation embryos. Semin. Cell Dev. Biol. 2017;. [PMID: 28577924]