Capturing E-cadherin
Lakshmi Ramachandran | 24 July 2014
Cells interact with each other and their environment through physical contacts known as cell adhesions. These complexes are imperative for normal embryonic development, maintenance of tissue integrity and signaling pathways. Their disruption is often observed in certain cancers, genetic diseases, cardiovascular disorders and Alzheimer’s disease. Therefore, understanding cell adhesions and the mechanisms that regulate their formation has been a major focus for researchers in recent years.
This work was led by Assoc. Prof. Virgile Viasnoff. (Engl W et al. Actin Dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat Cell Bio, 2014, 16:587-594).
Dynamics of cortical actin in the recruitment o cadherin at adhesion sites
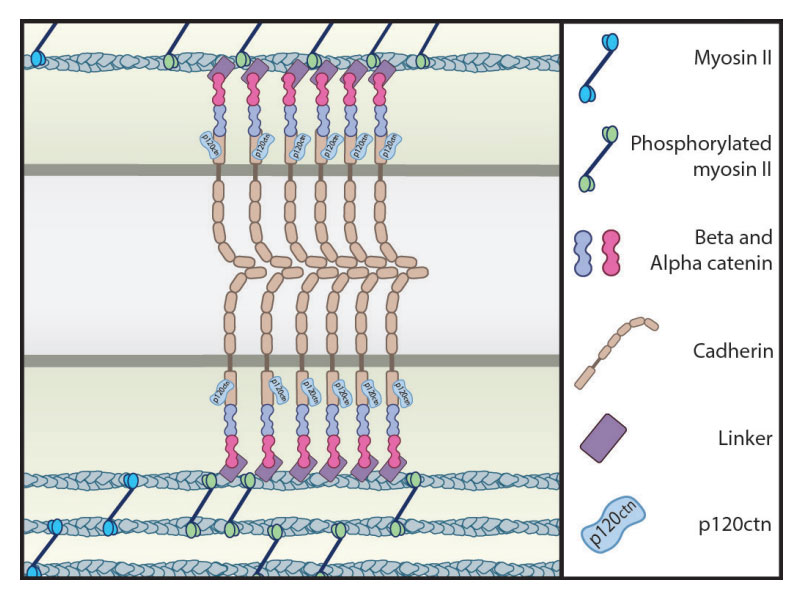
Cell adhesions are formed via the assembly of adhesion proteins, such as E-cadherin (E-cad) and α/β-catenin, at the cell membrane. These proteins are physically linked to an underlying cytoskeleton, which is a dynamic network of filaments predominately composed of a protein known as actin. This network constantly undergoes assembly and disassembly, and is also contractile, a property that is attributed to the presence of the motor protein, myosin II. The dynamic properties of the cytoskeleton enable the generation and transduction of force throughout the cell, including those that originate at cell adhesions.
Mechanical forces regulate cell adhesions
A question that has remained of interest to scientists is whether this signal transduction occurs one way, from the cell-adhesion into the cell, or whether it can feed back to affect the formation of the cell-cell adhesion site. Efforts to answer this question were often hampered by the presence of cell- matrix adhesions, which also detect and transduce mechanical force signals to influence the connected actin cytoskeleton.
Recently, researchers at the Mechanobiology Institute, Singapore overcame this challenge by developing a novel and robust assay that allowed them to see, through the microscope, the formation of cell-cell adhesion complexes at the interface of two floating and interacting cells. Imaging of the cell- cell contact surface, together with the measurement of forces borne solely by cell-cell adhesions in the absence of cell- matrix adhesions, revealed that indeed, cytoskeletal dynamics regulate the formation and stability of cell-cell adhesions.
Here, the accumulation of E-cad, as well as its organization into distinct clusters at the rim of the contact plane, resulted from a reduction in actin filament turnover at the cell- cell junction. These actin dynamics were mediated by the motor protein myosin II and this was confirmed when contractility was inhibited, which led to a reduction in both the concentration of E-cad and contact size.
Researchers develop a novel assay that allows them to see the formation of cell-cell adhesion complexes at the interface of two floating and interacting cells.
Although the actomyosin network has been implicated in many biological scenarios, from cell motility to muscle contraction, this is the first time it has been shown to play such a crucial role in the formation of cell-cell adhesions. In this case, a reduction in the turnover of the underlying actin leads to the sequestration of the E-cad and a proposed stabilization of interactions between E-cad molecules of opposing cells.
This scenario differs from the mechanism usually described, which suggests an increase in actin at the adhesion site occurs along with the recruitment of the scaffolding protein, vinculin. In this study however, vinculin was found to be evenly distributed around the contact plane instead of within the regions where E-cad was sequestered.
The transmission of force through cells is rarely one way. Once the cell has detected a mechanical signal, it is amplified via various signaling pathways, biochemical reactions and physical alterations to the cytoskeleton. Often these signals feedback to the very site where the initial signal was detected. As shown by Engl and colleagues, this appears to be an added layer of regulation that is crucial in the formation of cell adhesions. Increasing size and strength of the adhesion continues to increase tension in the cortical actin network, which in turn reduces actin turnover, enabling more E-cad to bind and allowing the adhesion to continue to grow in size and strength.