A Mechanical STOP Sign
Lakshmi Ramachandran | February 2016
A collaborative, interdisciplinary study between two research groups at the Mechanobiology Institute (MBI), NUS, has discovered the differential effects of extracellular matrix (ECM) topography on the proliferation of normal and cancerous cells. This work is published in Scientific Reports. [Chaudhuri PK et al., Topography induces differential sensitivity on cancer cell proliferation via Rho-ROCK-Myosin contractility, Sci Rep. 2016 Jan 22;6: 19672]
MBI Scientists reveal the effects of ECM topography on the poliferation of normal and cancer cells
The environment we live in has a deep impact on our lives. For example, changes in weather, region, or people can influence our moods and determine our activities. Similarly, at a microscopic level, changes in the nature of the environment around cells – the smallest functional units that make up our body, can determine whether a cell should divide, specialize or move.

Parthiv Kant Chaudhuri, a joint graduate student from the labs of Profs. Lim and Low, questioned whether such changes in ECM topography during cancer progression may also influence the proliferative potential of cancer cells.
The microenvironment of the cells is primarily the ECM, which is an extremely complex substance basically composed of water, proteins and polysaccharides. The ECM chemical composition, its mechanical stiffness, as well as topographical features like furrows, ridges or pores vary in different cells and tissues. It also changes with age, during wounding, and in cancer. Researchers have long sought to understand how such physical changes in the ECM correlate with cell behavior. Previous studies have largely looked at how the composition and stiffness of the ECM relates to cell behaviour, meaning that very little is known about the relationship between ECM topography and cell behavior.
Effect of topography on cell proliferation
In breast cancer, ECM topography changes during tumor progression as fibrous proteins like collagen organize and align, in contrast to their random organization under normal conditions. The aligned fibers enable the movement of cancer cells, fueling their ability to spread within the body. As cancer cells also possess limitless potential to divide and multiply uncontrollably, Parthiv Kant Chaudhuri, a joint graduate student from the labs of Prof. Chwee Teck Lim and Assoc. Prof. Boon Chuan Low at MBI, questioned whether such changes in ECM topography during cancer progression may also influence the proliferative potential of cancer cells.
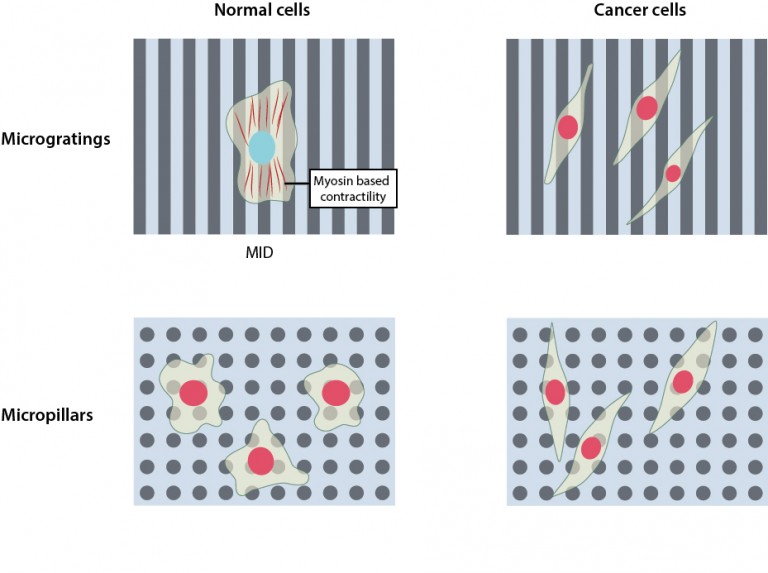
To address this question, the researchers artificially created two distinct types of ECM topographies, ‘micropillars’, mimicking the random organization of fibrous proteins observed under normal conditions and ‘microgratings’, resembling the aligned organization of collagen fibers as seen during breast cancer progression. Upon observing the proliferation of normal and cancer cells on these two topographies, they discovered that microgratings, which mimicked the cancer microenvironment, restricted the proliferation of normal cells, but not cancer cells. However this restriction in the proliferation of normal cells was not seen on micropillars, which provided an environment where both normal and cancer cells proliferated.
Revealed: a novel mechanism by which normal cells sense external topographic cues, restricting their proliferation to promote tumor growth and spreading.
Further investigation into what was causing the temporary dormancy of normal cells on microgratings led the research team to probe the role of the cell cytoskeleton, a dynamic and extensive network of filamentous proteins within cells that receives and transmits mechanical signals from the environment. The researchers discovered that on microgratings the cytoskeleton of normal cells is highly contractile, owing to the activity of a protein called ‘myosin’, which generates a mechanical signal that restricts their proliferation. They named this phenomenon ‘Mechanically-Induced Dormancy (MID)’.
This study reveals a novel mechanism by which normal cells sense external topographic cues and restricts their proliferation in an environment that promotes tumor growth and spreading. This finding provides an understanding of how the cell’s microenvironment plays a major role in maintaining normal tissue homeostasis during healthy conditions. Determining how tumor cells bypass this mechanism of MID may be paramount in the development of novel anti-cancer strategies that target cellular mechanisms altered by physical or mechanical cues during cancer progression.