A Link to Bacterial Gene Silencing
The charged H-NS linker is key to promoting DNA binding
Written by Andrew MS Wong, PhD | 18 Jan 2018 | Illustration by Diego Pitta de Araujo, PhD
Dr. Yunfeng Gao and colleagues from the lab of Prof. Linda Kenney at the Mechanobiology Institute have discovered that the linker in the bacterial nucleoid-associated protein H-NS has a vital role in promoting H-NS binding to DNA. Within the linker are 5 amino acids residues with a positive charge, and the electrostatic attraction from these residues is key to the initial interaction between H-NS and DNA. Once attached, H-NS can silence bacterial genes that cause virulence, and this silencing activity can be abolished by mutating the linker. This study was published in the Proceedings of the National Academy of Sciences.
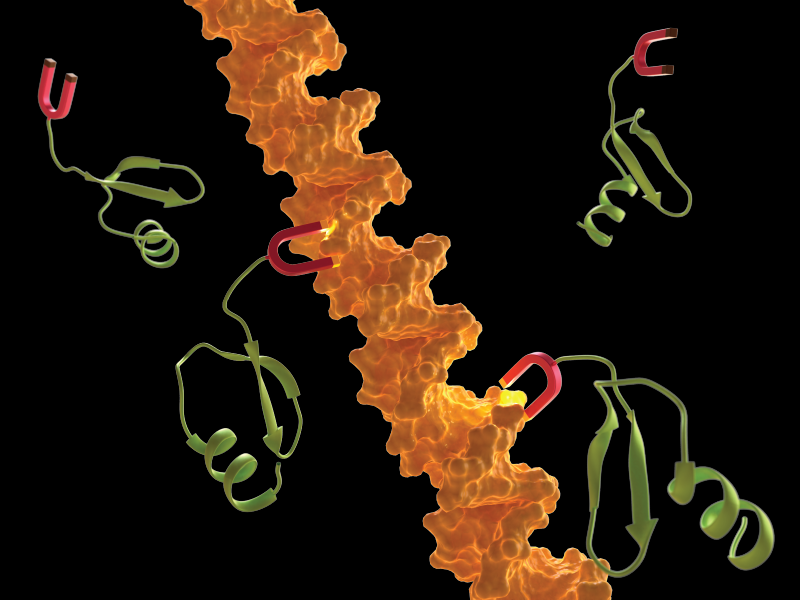
A stylized illustration of the linker (red) of H-NS protein (green) binding to DNA (orange).
The missing link explaining H-NS binding to DNA
Bacteria store their DNA in a structure called the nucleoid. Unlike animal cells, which store genetic information in the nucleus, the nucleoid does not have a membrane to encase the DNA. However, similar to animal cells, the length of bacterial DNA is much longer than the bacterial cell, approximately 500-1000 times the length of the cell. Therefore, the DNA needs to be compacted and packaged in order to fit inside the bacteria. This packaging is carried out by nucleoid-associated proteins (NAPs), which also have another important role in switching genes on and off. One specific NAP is the histone-like nucleoid-structuring protein H-NS that silences around 5% of the genes in the bacterial genome. Importantly, a subset of these genes are involved in virulence, so understanding how H-NS works could lead to new approaches for fighting bacterial diseases.
How does H-NS bind to DNA?
Structurally, H-NS is made up of two protein domains connected by a linker. One domain binds to DNA, and the other controls how H-NS connects other copies of itself. The linker is a region of 15 amino acid residues, the building blocks for proteins. Many multi-domain proteins are internally connected via linkers, which can also impart flexibility to large macromolecules. When H-NS binds to DNA it stiffens the DNA filament, similar to how a splint is used to immobilize a broken bone. This stiffened DNA cannot be accessed by proteins that read and decode the genetic sequence, thereby preventing gene expression. This mode of action is how H-NS acts as a gene silencer, but little was known about how H-NS to DNA binding was initiated.
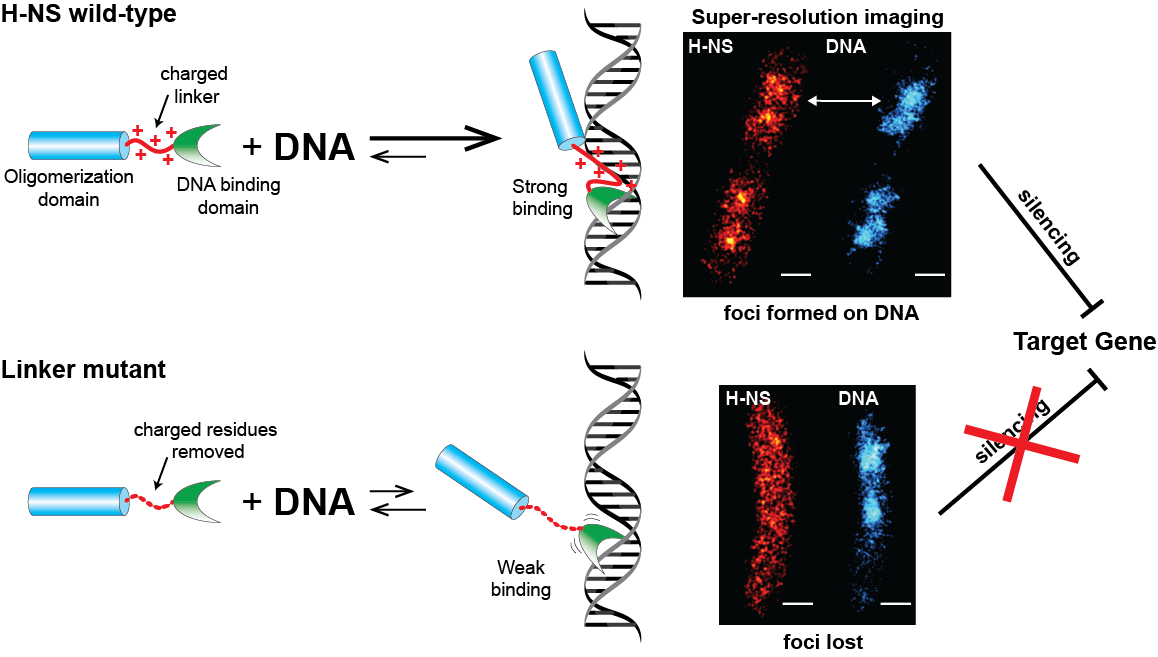
The charged linker of H-NS promotes strong binding of H-NS to DNA. H-NS foci formed on DNA can be observed using super-resolution imaging, and this binding results in gene silencing. When the linker is mutated by removing the charged residues, H-NS can only weakly bind to DNA, does not form foci, and is therefore unable to silence target genes.
However, when scientists split up the H-NS protein to study the two domains separately, they did not function as expected. Remarkably, the DNA-binding domain on its own was 2000 times less likely to bind to DNA than the full-length H-NS. As the self-binding domain does not affect DNA binding, a team of researchers led by MBI Principal Investigator Prof. Linda Kenney, theorized that the linker domain must be influencing DNA binding in some way.
In order to investigate this, the research team genetically altered H-NS to mutate or remove amino acids in the linker, and observed how this affected H-NS binding to DNA via super-resolution imaging and gene activity assays. Removing or mutating the amino acids in the linker resulted in an H-NS that had a reduced affinity for DNA, and was therefore less proficient at gene silencing. Super-resolution imaging revealed that normal H-NS could be observed as dense dots that associated with nucleoid DNA, but these dots dispersed when the linker was mutated, indicating that the mutant protein was unable to bind to DNA. Interestingly, mutating the H-NS linker did not lead to unravelling and relaxation of DNA as was expected. Unlike other NAPs, it appears that the primary function of H-NS is not compaction and packaging of DNA into the nucleoid.
Understanding how the H-NS linker is integral to H-NS mediated silencing of bacterial genes that cause infection could lead to new avenues for designing anti-bacterial treatments that could mimic the activity of H-NS.
The positive attraction between H-NS and DNA
Five of the amino acid residues in the linker have a positive electric charge, which means the linker has a net charge of +5. Given that DNA is negatively charged, perhaps H-NS could bind to DNA through electrostatic interactions. This was proven correct, as changing these charged residues into neutral residues disrupted H-NS binding activity. By carefully modifying individual residues, the research team discovered that a net charge of at least +4 was required for H-NS to bind to DNA and silence genes; increasing the number of positive residues above 5 led to impaired H-NS function. By running multiple computational simulations of the molecular interactions between H-NS and DNA, the researchers postulated that the electrostatic attraction from the linker was the critical first step in DNA binding, followed by H-NS sliding along the DNA until it finds a suitable target region for the DNA-binding domain. Without the charged residues in the linker driving the initial binding to DNA, H-NS was unable to function.
This study shows that the main role of H-NS is gene regulation and silencing, and not the expected role of DNA packaging. Importantly, the previously ignored linker region was essential for the silencing activity of H-NS. As many multi-domain proteins are connected via linker regions, this study highlights the need to consider all regions of the protein when investigating function. With this new understanding of how H-NS is able to silence bacterial genes that cause infection, these findings open up new avenues for designing anti-bacterial treatments that could mimic the activity of H-NS.