The mechanics of tissue development
The development of a multicellular organism occurs over a precise series of steps that are defined not only by genetic information, but by mechanical cues and the physical forces present in the cellular microenvironment.
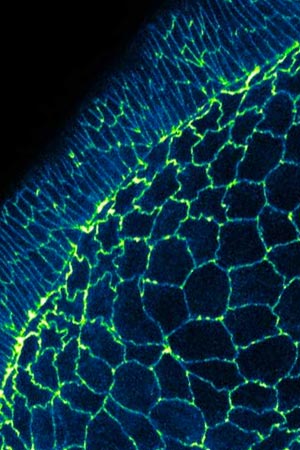
MBI researchers have discovered a new mechanism of cell boundary elongation. Read the full article: Elongation by Contraction.
The biophysics of development is being investigated at MBI, starting with a key aspect of the initial step in the process – zygote division. This often occurs asymmetrically so as to generate non-identical sister cells and enable cell diversification early in the development process. For symmetry breaking to take place, cells must become polarized, and this process is also being researched at MBI.
Also under investigation at MBI is the importance of forces that are generated during the extrusion of apoptotic cells from their surrounding tissue.
Cell apoptosis is crucial in development as it enables the size of the embryo, and the number of cells in each region, to be precisely regulated. Here, apoptosis balances out cell division. At the MBI, we investigate how cells achieve extrusion using actomyosin cables. These cables, which are formed within the dying cell as well as within neighboring cells, contract in what is known as the purse-string mechanism. This contraction generates a sufficient amount of force to squeeze the dying cells out.
In order to maintain tissue integrity, the empty spaces that result from cell extrusion must be filled. This is achieved using the same ‘purse-string’ mechanism of actomyosin contraction, with the cables in cells adjacent to the space contracting and pulling the cells towards each other, across the void.
As cells divide and proliferate, they also migrate and rearrange themselves to assume the correct position and shape within the developing embryo. Defects in cell migration can lead to severe embryonic malformations. Hence, MBI scientists are probing how cells migrate to precise positions reliably and reproducibly during development.
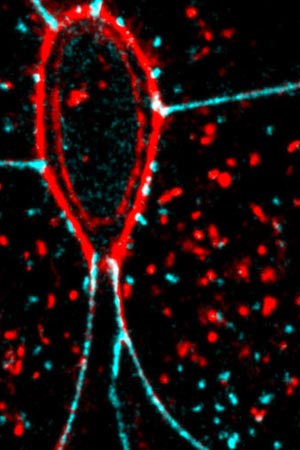
The purse-string (red oval) contracts to detach the dying cell from its neighbours. Read the full article: Letting Go.
Mechanics of cell migration in other critical processes like wound healing and cancer metastasis are also researched extensively at MBI with microfabricated substrates and traction force microscopy used to measure forces during migration. These tools allow us to closely mimic the 3D environment of the tissues in situ, which is crucial as it is known that the migration of cells in culture differs depending on the nature and stiffness of matrices used to grow them.
Modelling the theoretical
Tissue patterning, as well as the precise positioning of cells during development, is governed by signaling molecules known as morphogens. These molecules form a concentration gradient across the embryo, resulting in differential gene expression at spatial boundaries set by morphogen gradients. Some of these gradients appear to be optimized to minimize biological fluctuations and ensure robustness during development.
MBI researchers are trying to understand the noise minimization principles underlying robust embryo development with respect to tissue patterning and scaling through theoretical predictions and experimental biology.


