GAO Yunfeng
Alumni, Research Associate, Mechanobiology Institute, National University of Singapore
Transfer to Physics 31 Dec 2020
A link to bacterial gene silencing
The charged H-NS linker is key to promoting DNA binding
Gao Yunfeng
Alumni, Research Associate 2009-2020
Principal Investigator
I was trained as a molecular biologist in the Department of Biological Sciences, National University of Singapore. My project was a collaboration with the Department of Otolaryngology, focusing on characterization of novel allergens from house dust mites. During the study, I noticed that the local sequence and structure of a protein are so important that only a few amino acids changes in an allergen will induce severe allergic responses in patients. After receiving my PhD degree, I joined Dr. Ganesh Anand’s lab in the Department of Biological Sciences, NUS, to study the effect of protein structure and dynamics on its function. Currently, I am working with Prof. Linda Kenney in the Mechanobiology Institute and collaborating with Dr. Ganesh Anand to investigate the mechanism of EnvZ/OmpR mediated osmoregulation of ompC and ompF in E.coli.
Recent Research Interests
Salmonella is a common foodborne infection throughout the world. Two different diseases can be caused by Salmonella: gastroenteritis and life-threatening typhoid fever. In the constant battle between host defense systems and bacteria, Salmonella have acquired foreign genes by horizontal transfer which can increase the fitness of bacteria in changing environmental or growth conditions, e.g. inside the mammalian host cell. However, not all horizontally transferred genes benefit the recipient strain. To protect itself, Salmonella develops multiple defense systems, including the nucleoid-associated protein H-NS, a global transcriptional repressor, which serves as one of the first line of defense against horizontally acquired genes in Salmonella. Our first question is how does H-NS target and shut down its downstream genes?
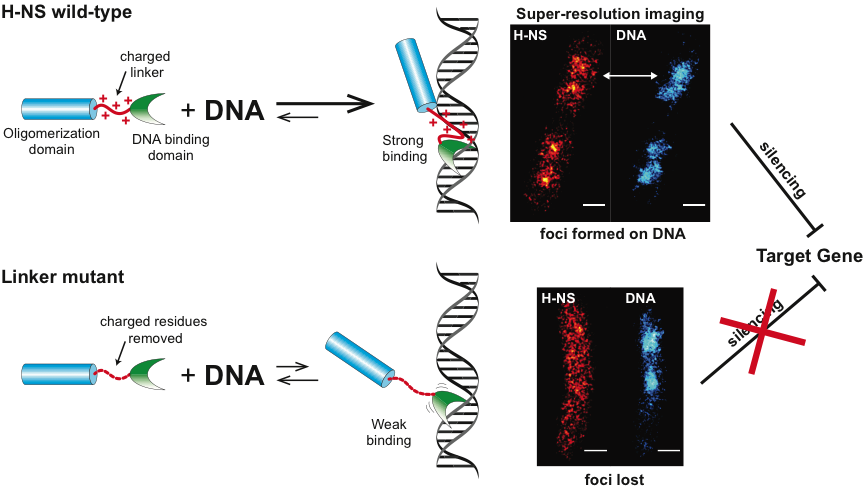
Our team discovered that this unstructured linker region plays an important and surprising role in promoting DNA binding.
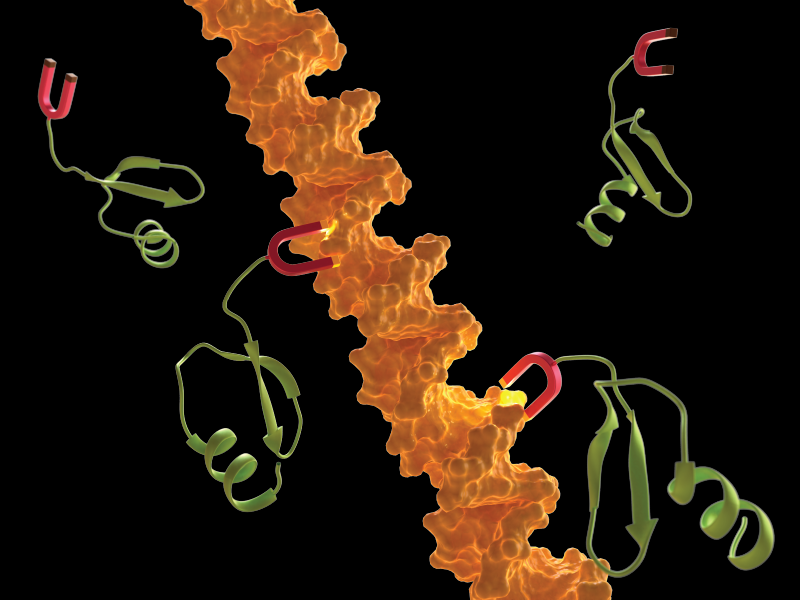
H-NS is present in many species of Enterobacteriaceae, including Salmonella and E. coli. It consists of two highly conserved domains, a N-terminal coiled-coil oligomerization domain and a C-terminal DNA-binding domain. Two distinct domains are connected by a disordered linker. This disordered linker has been overlooked in biology, because the conventional structure–function paradigm requires a fixed, 3D structure for function. We discovered that this unstructured linker region plays an important and surprising role in promoting DNA binding. Removing the linker led to weak DNA binding and relief of gene silencing. Super-resolution imaging revealed that only wildtype H-NS could form dense foci on the nucleoid DNA, but not the linker deletion mutant.
There are five conserved positively charged amino acid residues in the linker of H-NS across species. Given that DNA is negatively charged, we speculated that H-NS could bind to DNA through electrostatic interactions. Indeed, when these charged residues were modified to neutral residues, we observed that H-NS activity was significantly reduced. After carefully screening the amino acid residues in the linker, we discovered that a net charge of +4 or +5 was optimal for DNA binding and gene silencing, additional positive residues above 5 or less than +3 led to impaired H-NS function.
Taking advantage of the open lab concept of MBI, we were able to collaborate with researchers from other research teams with different scientific backgrounds for this project. Therefore, we were able to address the biological questions with different platforms, e.g. molecular genetics, protein-engineering, super-resolution imaging (Dr. Foo Yong Hwee), single molecule approaches and molecular dynamic simulations (Prof. Yan Jie’s group). We will extend this research to further investigate the regulation of Salmonella pathogenicity.
Publications
- Liew ATF, Foo YH, Gao Y, Zangoui P, Singh MK, Gulvady R, and Kenney LJ. Single cell, super-resolution imaging reveals an acid pH-dependent conformational switch in SsrB regulates SPI-2. Elife 2019; 8. [PMID: 31033442]
- Ghosh M, Wang LC, Huber R, Gao Y, Morgan LK, Tulsian NK, Bond P, Kenney LJ, and Anand GS. Engineering an Osmosensor by Pivotal Histidine Positioning within Disordered Helices. Structure 2018;. [PMID: 30503779]
- Gulvady R, Gao Y, Kenney LJ, and Yan J. A single molecule analysis of H-NS uncouples DNA binding affinity from DNA specificity. Nucleic Acids Res. 2018;. [PMID: 30239908]
- Mather AE, Phuong TLT, Gao Y, Clare S, Mukhopadhyay S, Goulding DA, Hoang NTD, Tuyen HT, Lan NPH, Thompson CN, Trang NHT, Carrique-Mas J, Tue NT, Campbell JI, Rabaa MA, Thanh DP, Harcourt K, Hoa NT, Trung NV, Schultsz C, Perron GG, Coia JE, Brown DJ, Okoro C, Parkhill J, Thomson NR, Chau NVV, Thwaites GE, Maskell DJ, Dougan G, Kenney LJ, and Baker S. New Variant of Multidrug-Resistant Salmonella enterica Serovar Typhimurium Associated with Invasive Disease in Immunocompromised Patients in Vietnam. MBio 2018; 9(5). [PMID: 30181247]
- Gao Y, Spahn C, Heilemann M, and Kenney LJ. The Pearling Transition Provides Evidence of Force-Driven Endosomal Tubulation during Salmonella Infection. MBio 2018; 9(3). [PMID: 29921673]
- Spahn C, Glaesmann M, Gao Y, Foo YH, Lampe M, Kenney LJ, and Heilemann M. Erratum to: Sequential Super-Resolution Imaging of Bacterial Regulatory Proteins, the Nucleoid and the Cell Membrane in Single, Fixed E. coli Cells. Methods Mol. Biol. 2017; 1624:E1. [PMID: 29151226]
- Gao Y, Foo YH, Winardhi RS, Tang Q, Yan J, and Kenney LJ. Charged residues in the H-NS linker drive DNA binding and gene silencing in single cells. Proc. Natl. Acad. Sci. U.S.A. 2017;. [PMID: 29109287]
- Spahn C, Glaesmann M, Gao Y, Foo YH, Lampe M, Kenney LJ, and Heilemann M. Sequential Super-Resolution Imaging of Bacterial Regulatory Proteins: The Nucleoid and the Cell Membrane in Single, Fixed E. coli Cells. Methods Mol. Biol. 2017; 1624:269-289. [PMID: 28842890]
- Foo YH, Gao Y, Zhang H, and Kenney LJ. Cytoplasmic sensing by the inner membrane histidine kinase EnvZ. Prog. Biophys. Mol. Biol. 2015; 118(3):119-29. [PMID: 25937465]