LOW Boon Chuan
Associate Professor, Mechanobiology Institute, National University of Singapore
dbslowbc@nus.edu.sg
Level 10 T-Lab
National University of Singapore
5A Engineering Drive 1
Singapore 117411
Research Program
The Cell-Matrix and Cell-Cell Mechanotransduction Group
The (BP)GAP Between Signaling Pathways
To grow or not to grow?
Too Deep To Divide!
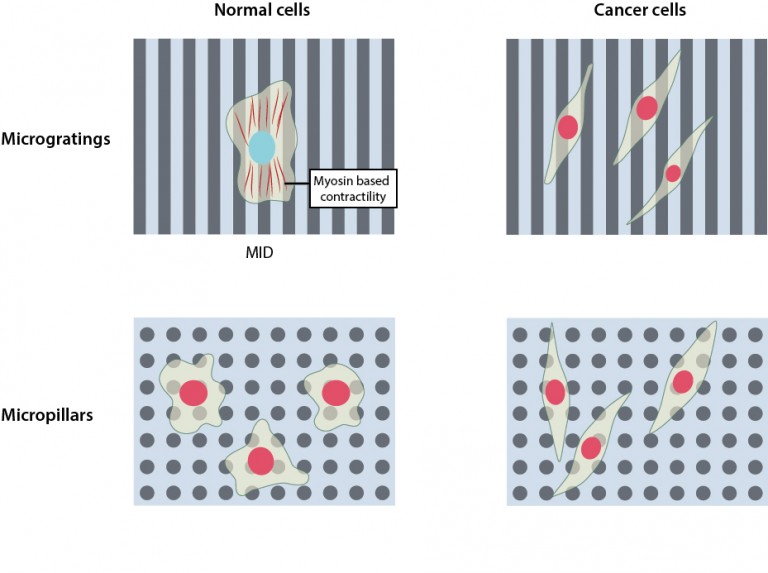
Molecular response to depth sensing differs in cancer cells
B(a)iled Out
Actin remodeling drives bile regurgitation during obstructive cholestasis
A Mechanical Stop Sign
MBI Scientists reveal the effects of ECM topography on the poliferation of normal and cancer cells
Regulating Neuron Growth
BNIP-H regulates neurotransmitter Acetylcholine
Rho Puts the Brakes on RAS-Driven Liver Cancer
Clues for targeting Ras-driven liver cancer through RhoA activation
Low Boon Chuan
Principal Investigator
Research Areas
To identify novel signaling proteins and protein domains that control cell morphogenesis, motility, differentiation and cell growth and tissue/organ development during normal and disease states. Areas of interest include cell signaling, domain-discovery, protein-protein interaction, structural biology, developmental biology, computational biology and mechanobiology.
Research Interests
Molecular recognition forms the basis for all cellular events- from a simple bimolecular enzymatic reaction to the cascades of multimeric protein complex in cell signaling. Fundamental to the structure and function of a protein is its ‘domain’- a discrete, minimal modular entity that constitutes one of the basic physical and functional unit of the polypeptide. This protein domain can either serve as a protein docking/interaction site or an active enzymatic unit. With the emphasis on functional genomics, it is important to address what role does each of these domains play and how their potential functions can be regulated across molecular, cellular and tissue levels.
One of the several protein domains that our group first identified and characterised is a novel protein domain termed BCH domain which play important roles in regulating cell growth/death, differentiation, migration, and tissue/organ development. Based on the prototypical BNIP-2 and BPGAP1 proteins, we show that distinct BCH domains could act as key modulators for Rho and Ras small GTPases as well as their immediate regulators such as guanine nucleotide exchange factors and GTPase-activating proteins. Current effort is geared towards understanding how cells and tissues respond to the dynamic forces and geometry in the environment both under the influence of the BCH domain as a versatile regulatory scaffold domain. These effects will be examined under normal and pathological conditions such as in cancers, neurological disorders, skin and heart diseases.
Biography
Assoc Prof Low left Kuala Lumpur, Malaysia for Dunedin, New Zealand in early 90’s to pursue his dream as a scientist. Having spent wonderful undergraduate and postgraduate years at the Department of Biochemistry, University of Otago, he joined IMCB, Singapore and then NUS, trying to figure out what exactly make cells work. Amazing as it is, we are still far from understanding the intricacies underlying these processes. Trained as a biochemist, practising mainly as a cell biologist now, and with exciting arrays of multi-disciplinary tools, his team and collaborators aim to systematically unravel some of the uncharted paths, and are ready to expect the unexpected.
Selected Publications
- Wong DCP, Pan CQ, Er SY, Thivakar T, Rachel TZY, Seah SH, Chua PJ, Jiang T, Chew TW, Chaudhuri PK, Mukherjee S, Salim A, Aye TA, Koh CG, Lim CT, Tan PH, Bay BH, Ridley AJ, and Low BC. The Scaffold RhoGAP Protein ARHGAP8/ BPGAP1 Synchronizes Rac and Rho Signaling to Facilitate Cell Migration. Mol Biol Cell 2023;:mbcE21030099. [PMID: 36598812]
- Wong DCP, Xiao J, Chew TW, Pan M, Lee CJM, Ang JW, Yow I, Thivakar T, Ackers-Johnson M, Lee NJW, Foo RS, Kanchanawong P, and Low BC. BNIP-2 Activation of Cellular Contractility Inactivates YAP for H9c2 Cardiomyoblast Differentiation. Adv Sci (Weinh) 2022;:e2202834. [PMID: 35975420]
- Meng Pan, Ti Weng Chew, Darren Chen Pei Wong, Jingwei Xiao, Hui Ting Ong, Jasmine Fei Li Chin, Boon Chuan Low1. BNIP-2 retards breast cancer cell migration by coupling microtubule-mediated GEF-H1 and RhoA activation. Science Advances 2020.
- , , , , , , , , , , Hiltonol, a dsRNA Mimic, Promotes NK Cell Anticancer Cytotoxicity Through TAZ Cytoplasmic Sequestration. Adv. Therap. 2023, 2300016. https://doi.org/10.1002/adtp.202300016
Recent Publications
- Ho CZ, Deng L, Picone R, Abderazzaq F, Flanagan N, Chua DZ, Low BC, and Wu SK. The Senescence-Associated Secretory Phenotype constitutes HIF-1α activation but is independent of micronuclei-induced cGas/Sting activation. Mol Biol Cell 2025;:mbcE24100445. [PMID: 41296514]
- Ho CZ, Lawther DB, Huang CB, Bharathkumar S, Beh SW, Poon EW, Lim PS, Lee KW, Felisha C, Tay SC, Yow I, Young JL, Wu SK, and Low BC. Protocol for AI-assisted quantitative analysis and setup of tumor spheroid invasion into tissue. STAR Protoc 2025; 6(4):104140. [PMID: 41075251]
- Sanz-Moreno V, and Low BC. Cell signaling across scales in health and disease. Curr Opin Cell Biol 2025; 97:102581. [PMID: 40946374]
- Xiao J, Ang JW, Zhong X, Wong DCP, T T, Yow I, Lee CJM, Foo RS, Kanchanawong P, and Low BC. Coordination of Focal Adhesion Nanoarchitecture and Dynamics in Mechanosensing for Cardiomyoblast Differentiation. ACS Appl Mater Interfaces 2025;. [PMID: 39778877]
- Shankar S, Liu Y, Tulsian NK, Low BC, Lin Q, and Sivaraman J. Insights into the regulation of CHIP E3 ligase-mediated ubiquitination of neuronal protein BNIP-H. PNAS Nexus 2024; 3(12):pgae536. [PMID: 39703232]
- Liu OX, Lin LB, Bunk S, Chew T, Wu SK, Motegi F, and Low BC. A ZO-2 scaffolding mechanism regulates the Hippo signalling pathway. FEBS J 2024;. [PMID: 39462647]
- Aureille J, Prabhu SS, Barnett SF, Farrugia AJ, Arnal I, Lafanechère L, Low BC, Kanchanawong P, Mogilner A, and Bershadsky AD. Focal adhesions are controlled by microtubules through local contractility regulation. EMBO J 2024;. [PMID: 38769437]
- Lee CJM, Autio MI, Zheng WH, Song Y, Wang SC, Wong DCP, Xiao J, Zhu Y, Yusoff P, Yei X, Chock WK, Low BC, Sudol M, and Foo RS. Genome-Wide CRISPR Screen Identifies an NF2-Adherens Junction Mechanistic Dependency for Cardiac Lineage. Circulation 2024;. [PMID: 38752370]
- Wu Y, Cheng J, Qi J, Hang C, Dong R, Low BC, Yu H, and Jiang X. Three-dimensional liquid metal-based neuro-interfaces for human hippocampal organoids. Nat Commun 2024; 15(1):4047. [PMID: 38744873]
- Shankar S, Chew TW, Chichili VPR, Low BC, and Sivaraman J. Structural basis for the distinct roles of non-conserved Pro116 and conserved Tyr124 of BCH domain of yeast p50RhoGAP. Cell Mol Life Sci 2024; 81(1):216. [PMID: 38740643]
Lab Members
Recent Lab Alumni
Xiao Jingwei
PhD Student, Class of August 2018, Low Group
Ajay Sanjay Tijore
Alumni, Low Group
Liu Xuan Olivia
Alumni, Low Group
Wong Chen Pei Darren
Senior Research Fellow, Low Group
Lin Bocheng Lester
Alumni, Low Group
Er Shi Yin
Alumni, Low Group
Chew Ti Weng
Alumni, Low Group
Pan Meng
Alumni, Low Group
Anushya Hariharan
Alumni, Research Associate, Low Group
Oh Dongmyung
Alumni, Low Group
Yao Mingxi
Alumni, Low Group