A Nervous Contraction
A collaborative study elucidates the mechanics behind a fruit fly’s ventral nerve cord condensation during development
When we think about an embryo in development, we often picture cells multiplying, tissues growing and organs expanding. Development is often seen as being synonymous with growth, but that is not always the case. In some instances, development can be reductive. Take for example how the webbings between the digits of a foetus’ hands disintegrate to produce free-moving fingers. Or how the final few segments of the spinal cord (known as our vestigial tail) fuse and reduce to make the tailbone.
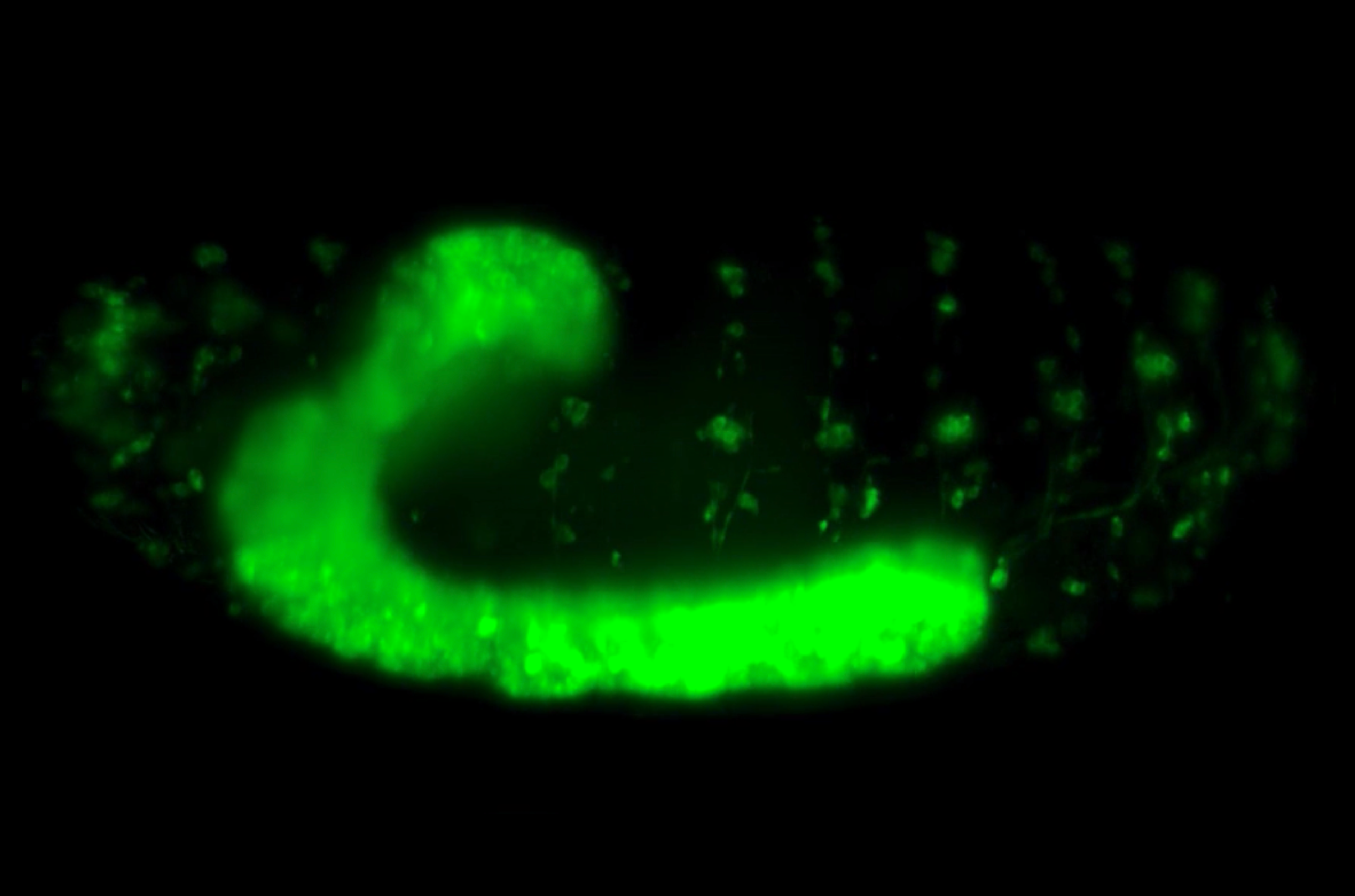
Another prominent instance of such development in nature lies in the nervous system of the fruit fly (Drosophila melanogaster). More specifically, their ventral nerve cord (VNC) — a structure functionally analogous to our spinal cord — was found to shrink into its final shape during embryonic development. Visually, the fly’s VNC is a long and ladder-like structure which also possesses a soft and bulbous texture. Biologically, that is due to having repeated segments of ganglia, which are dense clusters of interlinked nerve cells (or neurons). A connected pair of ganglia makes up a single segment of the VNC and each segment is linked to one another, just like how the rungs are linked together via the sides of the ladder.

3D visualisation of a section of the VNC
The genetics underpinning the development of the VNC is extremely well-studied, but the mechanical factors driving this phenomenon of down-scaling had yet to be uncovered. In 2018, MBI hosted Prof. Enrique Martin-Blanco from the Molecular Biology Institute of Barcelona, Spain, as a Visiting Professor with expertise in Drosophila development. Collaborating with Assoc. Prof. Timothy Saunders from MBI and his knowledge of live-imaging development processes and quantitative biology, they put together a team to explore the mechanical inner-workings of a contracting VNC. For a start, they needed to first establish some fundamental parameters surrounding the VNC and its condensation — such as how the VNC contracts, what its material properties are, what forces are at work, then how all these elements interplay.
Using live-imaging to visualise and measure the changing length and velocity of the VNC, they unveiled that the contractions did not take place in one sitting, but in three phases, with periods of rest sandwiched in between. The VNC is compressed from both ends towards a stationary, intermediary point between the thorax and the abdomen, like an accordion. However, these compressions do not occur simultaneously, but rather in an oscillatory or “see-saw” fashion. One end of the VNC first compacts itself a little towards the fixed point, followed by compaction on other end, then proceeding from the first end again, and so forth, creating a wave-like effect. Such extensive, repetitive action fostered the possibility that some sort of large-scale force and coordination must be involved.
Live-imaging of VNC contraction
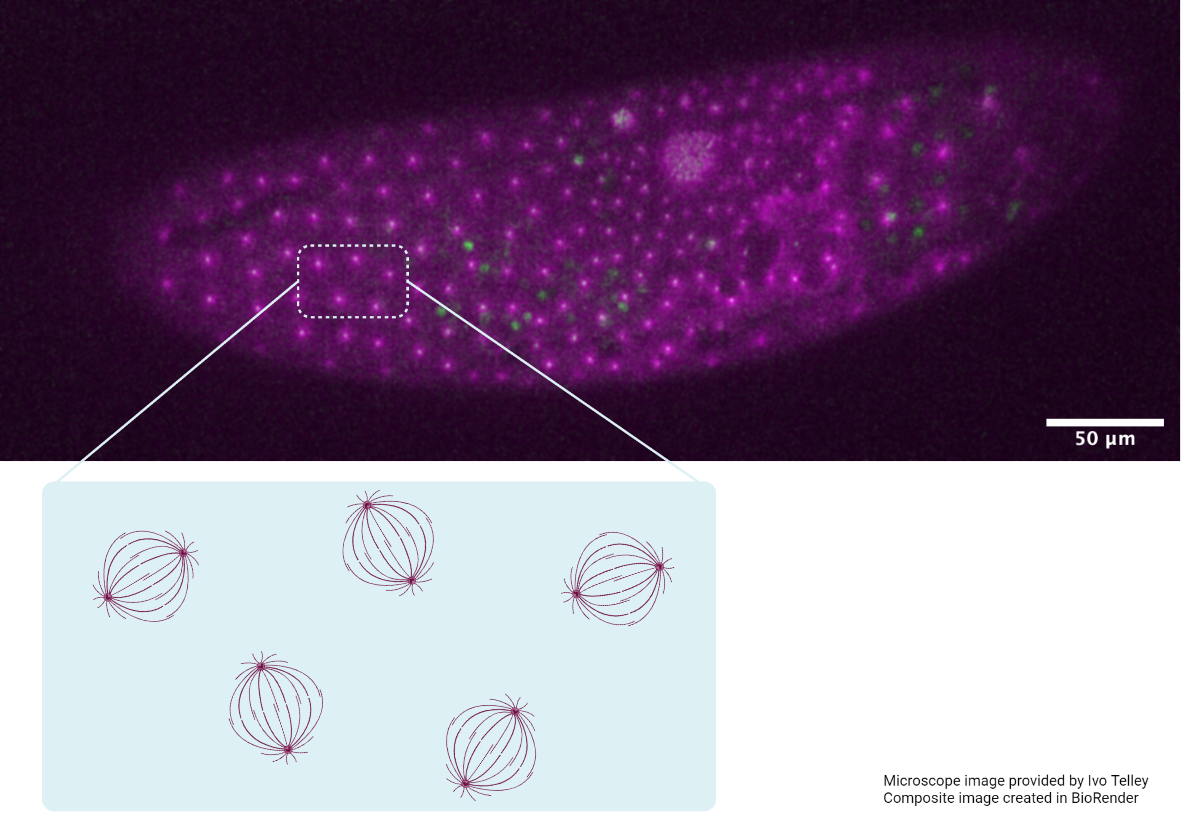
Having quantified the contraction throughout its morphogenesis, the team brought on board Prof. Jose Munoz from Universitat Politècnica de Catalunya, Barcelona, Spain, an expert in modelling and characterising tissues. Together, they investigated the material and mechanical properties of the VNC; Properties such as viscoelasticity and stiffness influence its contractility. Simply put, viscoelasticity refers to how much a material permanently changes shape or recovers its original form when a force is applied. And depending on the rate in which the material is deformed, the stiffness changes as well.
To do this, the team mapped tissue stress and used atomic force microscopy to measure the stiffness of the VNC. Although generally soft (or elastic), the stiffness of the VNC changed with position and increased with time. They discovered that the VNC was not mechanically homogenous throughout but compartmentalised into units that alternate in mechanical properties — i.e. having interchanging bands of stiffness and less-stiffness. Each unit was impervious to the next and could continue to contract independently even if severed from the rest. Most significantly, the alternating patterns of stiffness matched the distribution of cytoskeletal components in the VNC — active structures within the cells which can generate contractile forces and are responsible for cell shape and motion. This suggested that cytoskeletal activity could control VNC condensation. Following on from this, the team used their knowledge of VNC contraction and its mechanical properties to construct a theoretical model for describing the emergence of oscillations at the tissue level. Their simulations revealed that cytoskeleton dynamics were the strongest parameter for driving stable oscillation and successful condensation, confirming their experimental observations.
Putting this theory to the test, the team proceeded to interrogate if two types of cells in the VNC, neurons and glia — the support cells surrounding the neurons — and their cytoskeleton could influence the mechanical characteristics of the VNC and its contraction. Eliminating neurons or depleting their myosin II proteins, which generate the contractile force for cytoskeleton restructuring, only mildly affected oscillations and moderate disruption of VNC condensation. On the other hand, depleting the myosin II in glia resulted in weaker oscillations, and eliminating glia caused complete loss of oscillation and condensation failure. Taken together, VNC condensation appears to be largely driven by the compressive action of the glias, but also requires the coordinated contractile efforts of both glia and neurons for the large-scale progression of force throughout the entire VNC. The ‘see-saw’ oscillatory wave characteristic of VNC condensation could result from a slight delay in force transmission due to friction between glia and neurons.
This paper demonstrated how mechanical movements can generate oscillatory contractions for the condensation of nervous tissue during development. Interdisciplinary science, in this case, a collaboration between developmental biology, biophysics, and theoretical modelling, has revealed new dimensions for understanding developmental processes that depart from classical perspectives focusing more on cell growth.