Signaling in cell fate specification
Both when and where matter
Written by Sruthi Jagannathan | Illustration by MELANIE LEE | October 2018
The determination of progenitor cell fate is a fundamental process during embryo development. Signaling molecules, known as morphogens, instruct cells in fate determination. However, how such information is interpreted reliably within complex three-dimensional tissues during development and repair remains unknown. In their latest work, MBI PI Assistant Professor Timothy Saunders and graduate student Jianmin Yin have shown how the processes of morphogen signaling and cell migration interact to build the muscles around the spinal cord in vertebrates. These findings are published in Developmental Cell.
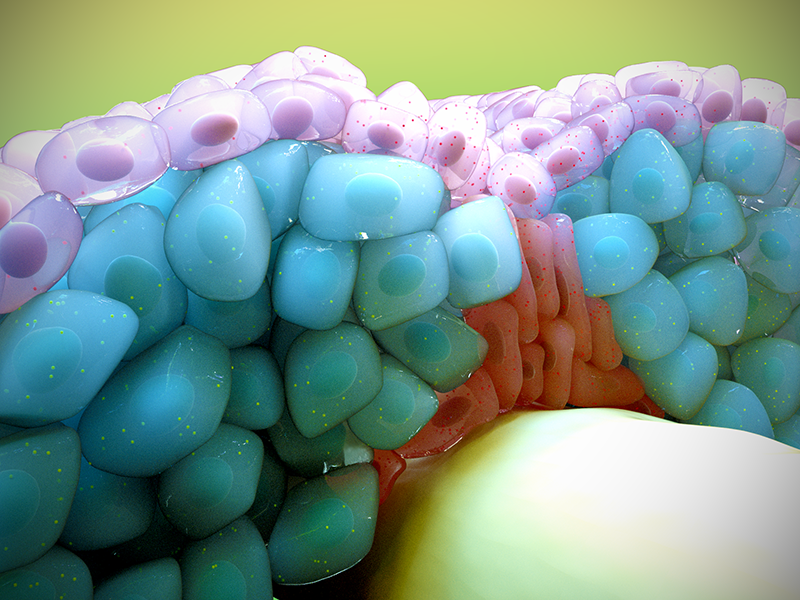
An artistic depiction of how fast (blue) and slow (purple) muscle progenitor cells migrate in response to morphogenic signals (shown as speckles) during skeletal muscle development.
Spatiotemporal control of morphogen signaling during skeletal muscle development
Our body functions as one large living machine, somehow incorporating behavior from trillions of cells. All of these cells need to adopt a specific fate in order to perform their function. A major challenge in developmental biology is trying to understand how the numerous cell fates (e.g. nerve cells, liver cells, muscles) are determined. When cell fate specification goes awry, it leads to diseases such as birth defects and cancers.
What controls the specification of progenitors into distinct cell types? This question can be answered by envisaging the early embryonic tissues as a highly dynamic microsystem, where progenitor cells are exposed to a plethora of signals (chemical, mechanical, or electrical) from their surroundings. Cells respond to the incoming signals in several ways- they change shape, proliferate, move to a new location, or even attain identity as a specific cell type. Despite the complexity of the system, embryo development usually proceeds through a precisely choreographed sequence of events, which suggests that cells have in place a robust way of integrating inputs from multiple signaling systems.
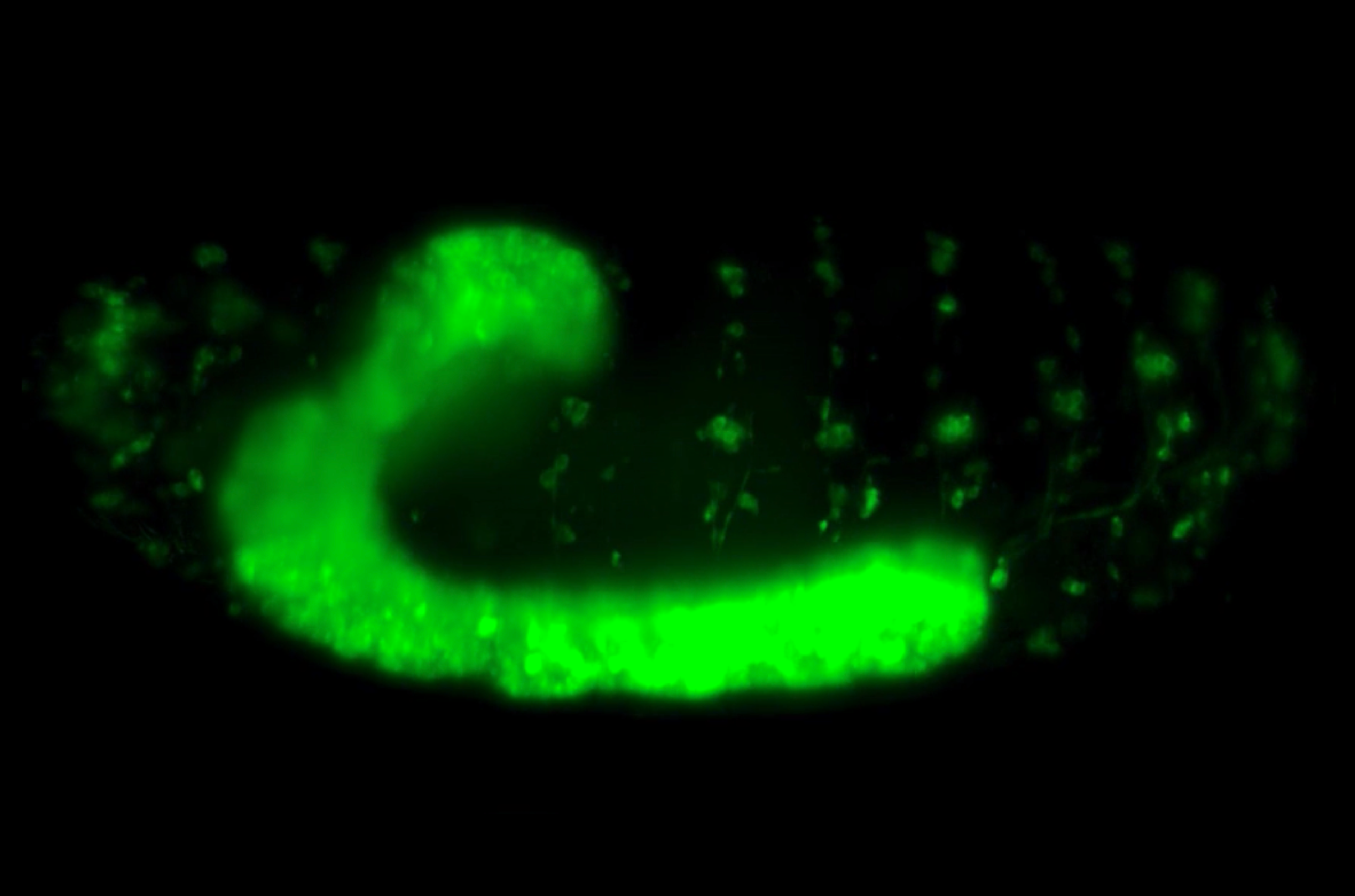
By using confocal and light sheet microscopy techniques to visualize live the process of muscle formation in Zebrafish embryos, Yin and team have revealed how muscle progenitor cells migrate in response to morphogen signaling during the development of slow and fast skeletal muscle fibers.
How this happens in real-time, that is, how multiple signaling systems are precisely coordinated during embryonic events, particularly during cell specification, was the focus of this study led by Jianmin Yin, a graduate student at the Mechanobiology Institute, National University of Singapore, under the guidance of Principal Investigator Assistant Professor Timothy Saunders.
Using confocal and light-sheet microscopy techniques that allowed the live imaging of muscle formation in Zebrafish embryos, the researchers studied the specification of progenitors into skeletal muscle tissues. Muscle cells are responsible for all types of body movements and derive from specific progenitor cells early in development. They are formed as two subtypes- fast and slow muscle fibers. Fast twitch muscles are powerful but have low endurance whereas slow twitch muscles have long endurance but less power. These muscle types are determined by two major signaling systems- Fgf and Shh- both of which have well-established roles in cell specification.
Jianmin, along with collaborators from the lab of Professor Philip Ingham at the Nanyang Technological University, tracked a group of progenitor cells during their specification into fast and slow muscle fibers. The slow muscle fibers are the first to evolve from a certain group of progenitors in response to Shh- a ligand produced by the notochord. This is followed by the generation of fast muscle fibers from a separate group of progenitors in response to a different signal, Fgf. As the progenitors forming fast muscle fibers elongate, they displace most of the slow muscle fibers away from the notochord, where they are no longer activated by Shh signals.
When this displacement does not occur at the precise time, the slow muscle progenitors undergo prolonged Shh activation, which leads to their development into muscle pioneers. Pioneer cells do not move away from the notochord; instead they specialize into slow twitch muscle fibers that stay near the notochord and support the development of later-forming muscles and motor neuron growth cones.
Based on the findings, they proposed a novel role for Fgf during skeletal muscle development: it indirectly controls the development of slow muscle fibers by limiting the duration for which slow muscle progenitors are exposed to Shh signaling, rather than directly signaling to slow muscle fibers.
The developing embryo is a complex and dynamic living system. How cells respond to the multitude of signals converging on them dictates whether the developmental programs proceed in an orderly fashion, eventually leading to a healthy individual. Right from acquiring cell identity, which is the basis for the robust functioning of all multicellular organisms (including humans), all cellular and tissue level events are closely controlled by the integration of multiple signaling systems.
The findings from the study further substantiate this fact by revealing how multiple signaling systems are precisely coordinated during skeletal muscle specification in developing embryos. Here, the researchers describe a space and time controlled system, wherein the fate of the progenitors is determined not just by their exposure to signals arising from neighboring tissues, but also by the duration of exposure to the signal. This knowledge can be useful in understanding how several other cellular events, all of which take place amidst a similarly complex and dynamic signaling environment, are controlled robustly during embryo development.