Too Deep to Divide!
Molecular response to depth sensing differs in cancer cells
Lakshmi Ramachandran, PhD and Steven Wolf, PhD. Illustrations by Diego Pitta de Araujo, PhD | Aug 2017
In research recently published in the highly regarded journal ACSNano, MBI graduate student Parthiv Kant Chaudhuri, has shed light on how topographical features found on secondary tumour sites such as bone, influence mechanisms within the cellular machinery that drives the growth and division of cells. This work has been published in ACSNano. (Parthiv Kant Chaudhuri, Catherine Qiurong Pan, Boon Chuan Low, and Chwee Teck Lim. Differential Depth Sensing Reduces Cancer Cell Proliferation via Rho-Rac-Regulated Invadopodia, ACS Nano, 2017, 11 (7), pp 7336–7348 DOI: 10.1021/acsnano.7b03452).
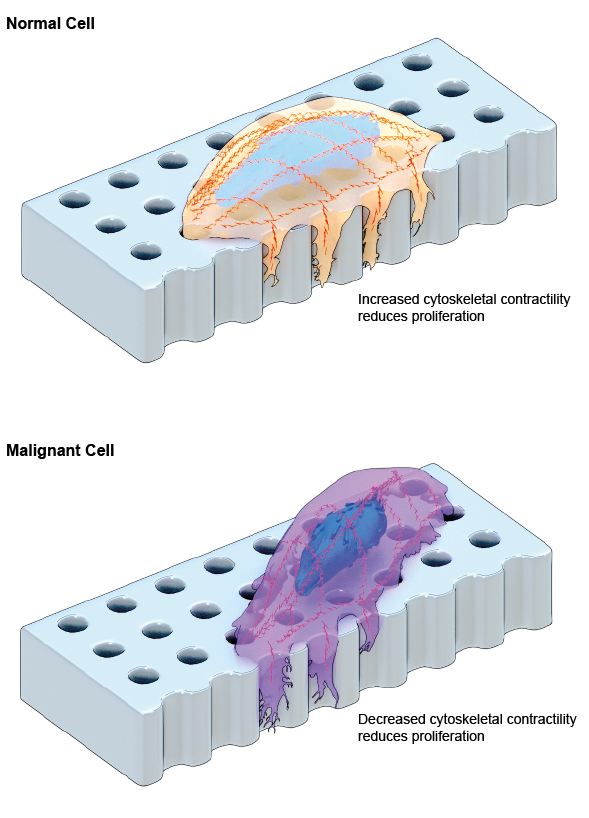
Schematic depiction of normal and malignant cells extending finger-like protrusions called invadopodia that can sense the depth of underlying pores on a fabricated surface that mimics the bone. Depth-sensing causes MID or reduced proliferation in both cells. However, the underlying mechanisms that causes MID in both cells are different and opposing: increased contractility of the actin cytoskeleton in normal cells vs. decreased contractility in malignant cells.
Pore depth of bones determines cell proliferation potential
Cancer is a disease that is characterized by the uncontrolled growth of cells. In some cases, cancer cells can spread from the primary tumour location, for instance, the breast, to secondary locations such as bone, brain, liver or lung. This occurs through a process known as ‘metastasis’ and is often attributed to a poorer prognosis for patients with advanced stage cancers. When cancer cells become metastatic, they not only gain the ability to move to regions of the body that are physically and biochemically distinct from the original tumour, but can adapt to these new conditions. In some cases, cells can move between environments of completely opposing properties. For example, cells that normally grow in the soft breast tissue may establish a secondary tumour within the much harder environment of the bones. In these cases, the physical properties of the new regions dictate the behaviour of the cancer cells, and promote or halt their growth and division, or cause them to move on to other sites.
How cells sense depth and halt cell proliferation
Now, in research recently published in the highly regarded journal ACSNano, MBI graduate student Parthiv Kant Chaudhuri, has shed light on how topographical features found on secondary tumour sites such as bone, influence mechanisms within the cellular machinery that drives the growth and division of cells. Specifically, the researchers analysed the mechanical effect of bone pore dimensions on the dynamics of the cytoskeleton during the proliferation of normal vs cancerous breast cells.
In earlier work from the MBI, the same researchers showed how features that are commonly encountered by cells, such as furrows, ridges or pores, can affect the proliferation of cells. Here, a phenomenon called Mechanically-Induced Dormancy (MID), was described, where normal breast cells, but not breast cancer cells, stopped dividing on an artificially created topography that mimicked the breast cancer microenvironment. This discovery opened up the possibility of identifying novel anti-cancer strategies that target cellular mechanisms altered by mechanical cues during cancer progression.
Anomalies in sub-cellular behavior may provide novel targets for new anti-cancer therapies, and be useful in preventing cancer cells from adapting and growing in new areas of the body.
To further their research on MID, and understand how the physical properties of bone influence breast cancer cell growth, the researchers fabricated pits of varying but defined width and depth, to mimic the porous matrix of the bone. Normal and malignant breast cells were grown on these micropits and were analysed by confocal microscopy. The team noted how the cells sent out finger-like protrusions into the micropits. They characterized these protrusions as ‘invadopodia’ which are cellular structures rich in a cytoskeletal protein called actin, that are put forth by cancer cells to enable sensing and degradation of the cancer cell’s microenvironment.
The researchers noted that at a specific depth, the sensing carried out by invadopodia initiated a halt in cell proliferation. This was observed in both cancer and non-cancer cells and was similar to the previously described phenomenon of mechanically induced dormancy (MID).
Although the response of both cell types was to halt their growth, it remained possible that the molecular mechanisms driving MID differed between the two cell types. Such differences would be consistent with what is known about cancer cell behaviour; that they often display erratic or uncontrolled protein activity in response to otherwise normal stimuli.
Indeed, when the researchers examined the molecular mechanism at play, they found that the cancer cells initiated MID by reducing the contractility of the cytoskeleton, which was in contrast to the normal cells, where contractility was increased. Furthermore, they found that the inhibition of contractility using chemical inhibitors further reduced the proliferation of cancer cells.
The findings from this study highlight the importance of understanding how cancer cells respond, at the sub-cellular level, when they relocate and encounter new and foreign environments. Here, the molecular responses to depth sensing differed from normal cells even though the ultimate outcome for both cells types was a halt to their proliferation. Such anomalies in sub-cellular behavior may provide novel targets for new anti-cancer therapies, and be particularly useful in preventing cancer cells from adapting and growing in new areas of the body.