The Nano-Heartbuilder: BNIP-2 Influences Mechanosensing in Cardiomyoblast Differentiation
Findings identify gap in understanding precise molecular mechanisms that regulate focal adhesion dynamics during heart development.
Researchers from the Low Lab at MBI discover a crucial role for the scaffold protein BNIP-2 in orchestrating focal adhesion dynamics during early heart development, offering new insights into heart regeneration strategies. This study was published in ACS Applied Materials and Interfaces. https://doi.org/10.1038/s44318-024-00114-4
A study conducted by Jingwei Xiao from the lab of Assoc Prof Low Boon Chuan at the Mechanobiology Institute, National University of Singapore, has revealed how the structural organisation and signaling pathways at focal adhesions – protein complexes formed at the interfaces of cells and surrounding tissues – are regulated during early heart development.
The human heart begins forming as early as the third week of fetal growth, when embryonic stem cells differentiate into specialised heart cells called cardiomyocytes. This process, like many fundamental biological functions, relies heavily on a form of cellular communication known as mechanosignaling – a dynamic exchange of mechanical and biochemical signals between cells and their environment.
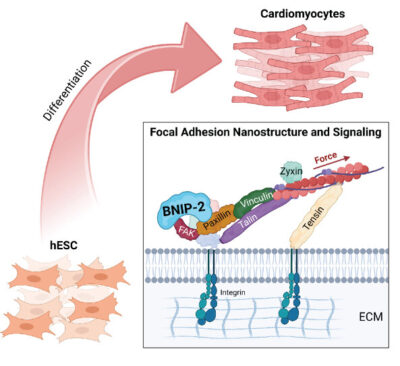
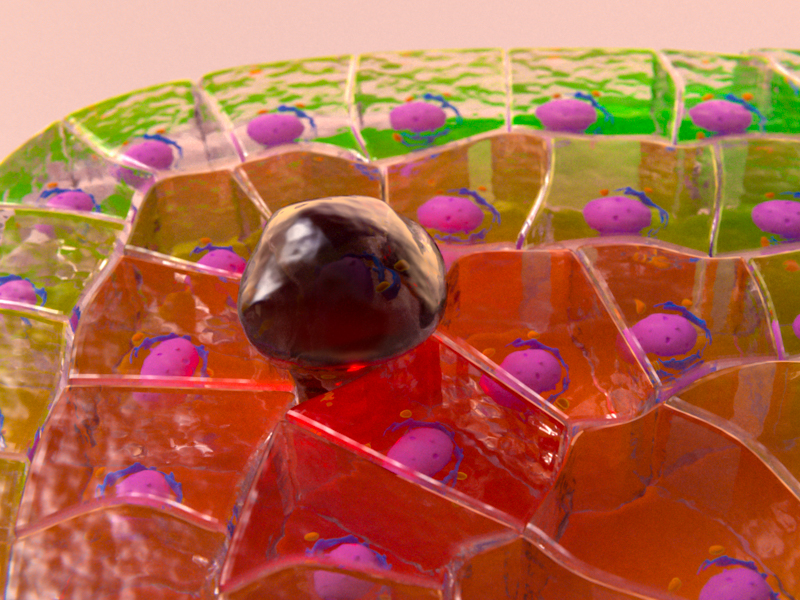
Graphic depicting how BNIP-2 plays a pivotal role in regulation of focal adhesions. Image credit: https://doi.org/10.1021/acsami.4c15459
At the core of this process are focal adhesions, which act as mechanical anchors and signaling hubs. The contractile forces generated by the sliding action of actomyosin filaments against the surrounding tissue – depending on stiffness and architecture – are converted into biochemical signals that guide cell growth, division, migration and tissue organization.
Mechanosignaling is particularly crucial during heart development, as it governs how stem cells evolve into cardiomyocytes and other supporting cells, including cardiac smooth muscle and endothelial cells. Despite its importance, the understanding of the precise molecular mechanisms that regulate focal adhesion dynamics during heart development is poorly understood. Identifying these key molecular events is crucial for advancing cardiovascular research, since cardiomyocytes renew at a very low rate throughout adult life and several debilitating diseases are associated with cardiomyocyte loss.
The knowledge gap was the focus of Jingwei Xiao’s study, conducted in collaboration with researchers from MBI and the NUS Yong Loo Lin School of Medicine. The team identified a pivotal role for a protein called BNIP-2 in controlling focal adhesion dynamics during early heart development.
Buillding on earlier research from the Low lab – which showed BNIP-2’s vital role as a scaffold protein in driving cardiac muscle differentiation by activating signaling proteins RhoA and YAP – the new study explored how BNIP-2 influences the nanostructural organisation of focal adhesions.
Scaffold proteins like BNIP-2 serve as docking platforms that bring together multiple proteins in close proximity, so they can physically bind to each other and activate a cascade of chemical reactions important for specific cellular functions.

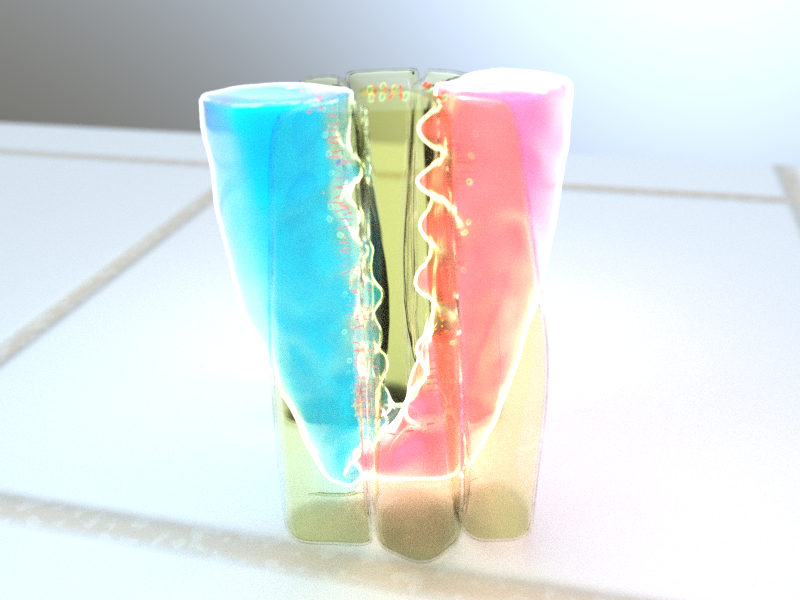
Schematic model of BNIP-2 scaffolding FAK to promote cardiomyoblast differentiation. The authors propose that BNIP-2 coordinates the integration of FAK, paxillin, and vinculin, leading to the activation of FAK on paxillin signaling, initiating cardiomyoblast differentiation. Image credit: https://doi.org/10.1021/acsami.4c15459
Using a scanning angle interference microscopy (SAIM), a cutting-edge imaging technique that provides nanometre-level resolution, the researchers generated images of focal adhesion proteins in the presence and absence of BNIP-2. They found that in the absence of BNIP-2, the typical organisation of these proteins was disrupted. Specifically, focal adhesion kinase (FAK) – which phosphorylates and activates several proteins for downstream chemical reactions – was mispositioned. This disruption prevented the activation of another signaling protein called paxillin and in turn, the recruitment of vinculin, another critical focal adhesion component.
As a result, cells formed fewer but larger focal adhesions and generated lesser contractile forces. This disrupted the cells’ mechanosensing abilities and hindered their ability to differentiate into cardiomyocytes.
Given the global upsurge in mortality and morbidity from heart diseases, a major part of cardiovascular research is focused on improving the self-repairing ability of heart cells and regaining heart function. This study offers a significant step forward, as it revealed the precise role of focal adhesions in heart cell differentiation.
By identifying the BNIP-2 protein as a central mechanosignaling hub for mechanically-induced cell differentiation, the study provides a foundation for future efforts that promote heart repair and rejuvenation.