The mechanics of cancer growth
How cancer cells spread in response to physical cues from their surroundings
Written by Sruthi Jagannathan | Illustration by MELANIE LEE | October 2018
The ability of cancer cells to spread or metastasize contributes to the high mortality rates of the disease. How cancer cell behavior – their ability to proliferate and migrate – is influenced by external signals, in particular, physical cues, was summarized in a recent review paper authored by Dr Parthiv Kant Chaudhuri, along with Principal Investigators Professor Chwee Teck Lim and Associate Professor Boon Chuan Low, Mechanobiology Institute, National University of Singapore. The review was published in Chemical Reviews. The review has also been recommended in F1000Prime as a paper adding significance to the field.
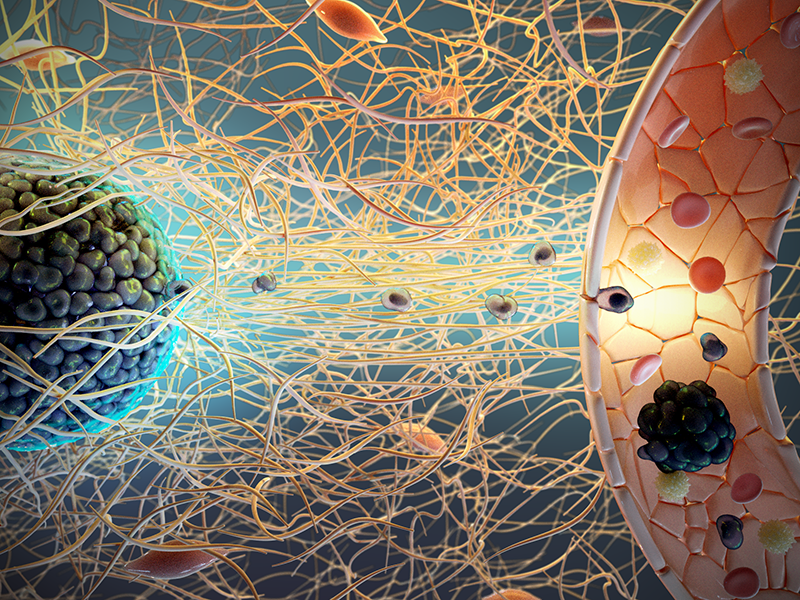
An artistic depiction of how cancer cells metastasize amidst dynamic physical properties of their environment.
Cancer metastasis depends on the physical properties of its surrounding tissue
The ability of cancer cells to spread inside the body is what make cancers one of the most devastating and dreaded diseases worldwide. This spreading – known as metastasis – starts with a few cancer cells breaking free from the primary tumor site, after which they can migrate around the body via the blood stream and infiltrate distant secondary tissue locations.
However, not all cancer cells spread alike. Where, and to what extent they spread is distinct for each cancer type and is determined by multiple factors. For instance, breast cancer cells spread to multiple locations including bones, lungs, brain, and liver, while prostate cancers usually spread only to bones. Similarly, the time-scales of cancer reoccurrence at a secondary location can vary dramatically. Breast cancers are known to recur years or sometimes decades after their first detection, whereas lung cancers spread within months of their first occurrence.
So, what causes differences in the metastatic potential of different cancer types? The answer lies in the dynamic environment of the cancer cells, which constantly present to cells a plethora of physical and biochemical signals. The signals interact closely with cells to produce complementary or antagonistic effects that together shape cancer cell behavior, such as their ability to proliferate or invade other tissues. These ideas, and the mechanisms underlying them, was the central theme of a recent review paper authored by Dr Parthiv Kant Chaudhuri and MBI Principal Investigators Boon Chuan Low and Chwee Teck Lim. The review specifically focuses on the physical signals that cancer cells are exposed to in their three-dimensional microenvironment and summarizes how these signals are relayed to the nucleus via biochemical signaling pathways, and how this controls cancer cell behavior.
Physical properties influence disease progression in a cancer type-specific manner
Any physical property of the surrounding tissue- be it stiffness, shape, or the amount of force that it exerts on cells – acts as a signal that can directly impact cellular behavior. For instance, healthy cells and cancer cells respond differently to tissue stiffness; healthy cells stop proliferating on softer tissues, whereas cancer cells are non-responsive to such physical signals and keep proliferating. However, even this cellular response to tissue stiffness varies amongst cancer types – while ovarian cancer cells prefer to proliferate on softer tissues, glioblastoma cells show increased proliferation on stiffer tissues.
Physical signals from the cellular microenvironment closely interact with cancer cells and shape their behavior, such as their ability to proliferate and invade other tissues.
Similar to stiffness, tissue structure is known to have varied effects on healthy vs cancerous cells. Healthy cells respond to a variety of structural cues in their surroundings by controlling their proliferation rates. This phenomenon is now referred to as mechanically-induced dormancy. On the other hand, cancer cells are able to bypass such regulatory cues and keep proliferating. This explains why some cancer types are able to spread into the bone, which has a diverse architecture of pores and pits that have varying depths. Even during invasion through dense matrix, the structural confinements of the tissue do not limit the proliferation of cancer cells, whereas healthy cells stop dividing under such conditions. This is due to the fact that cancer cell nuclei are softer and therefore more deformable than the nuclei in healthy cells. Nuclear deformations result in altered genetic programs, leading to an increase in the division of cancer cells. Other physical signals, such as compressive forces from surrounding tissues and shear stress from fluid flow around tissues, are known to affect cancer cell behavior, especially their ability to proliferate – in a cancer type dependent manner.
From sensing signals to eliciting responses
Many of the behavioral responses of cancer cells to physical signals from their surroundings depend on the mechanical properties of their nuclei. Cancer cell nuclei contain low levels of a particular protein called lamin, which forms a filamentous framework in the nucleoplasm. The absence of a well-established lamin filament skeleton means that cancer cell nuclei are softer than the nuclei in healthy cells, and subsequently more prone to damage or structural alterations induced by physical signals.
So, how do physical signals from outside the cell reach the nucleus? Several cellular components and molecular machineries come together at various levels to take part in the mechanotransduction pathway. At the cell surface, physical signals are sensed by large molecular machineries, including integrin-based cell-matrix adhesions and cadherin-based cell-cell adhesions. Beneath the cell surface, these molecular complexes are coupled to the cytoskeleton, primarily the actin filaments. The actin filaments, which are initially strewn across the cytoplasm, remodel into higher-order structures in response to physical signals. Actin remodeling generates forces, which are then transmitted across the cell as well as to the tissue surroundings. Deep inside the cell, actin structures are physically associated with the nucleus via LINC (Linker of Nucleoplasm and Cytoplasm) protein complexes. Therefore, a continuous structural link is established through which physical signals from outside the cell can reach the nucleus.
In addition to their direct transmission across cytoskeletal-nuclear linkages, physical signals can also activate one or more biochemical signaling pathways in the cytoplasm. Progressing via a cascade of protein interactions, these pathways eventually result in the movement of certain proteins – known as transcription factors- into the nucleus. Inside the nucleus, physical signals and biochemical factors directly influence genome programs. Physical forces alter the way chromosomes are spatially arranged inside the nucleus; this exposes attachment sites for transcription factors that are transported into the nucleus. The binding of transcription factors to specific chromosomal regions drives the expression of genes related to cancer cell proliferation and metastasis.
Understanding how the physical-chemical environment of cancer cells directly impacts their behavior is only the first step. The challenge for the future will be how scientists can harness mechanical principles to design new diagnostic or therapeutic approaches to complement the existing clinical treatments for different types of cancers.