Rac1-Regulated Cadherin Clusters Mark Naive Stem Cells
The findings broaden current understanding of the maintenance and architecture of ground-state pluripotent stem cells.
Researchers from the Kanchanawong Lab at MBI reveal non-junctional cadherin complexes that are governed by Rac1 activity, as a hallmark structure of naive stem cells.
Scientists at the Mechanobiology Institute, National University of Singapore, have uncovered a distinctive internal “signature” that marks stem cells in their most primitive state—and discovered that this signature is regulated by a key molecular switch, Rac1. The study, published in the Journal of Cell Science and led by Shiying Liu from the Kanchanawong lab, provides a new way to distinguish ground-state pluripotent stem cells (PSCs) from more differentiated states and offers insights into how cellular structure helps preserve their flexibility.
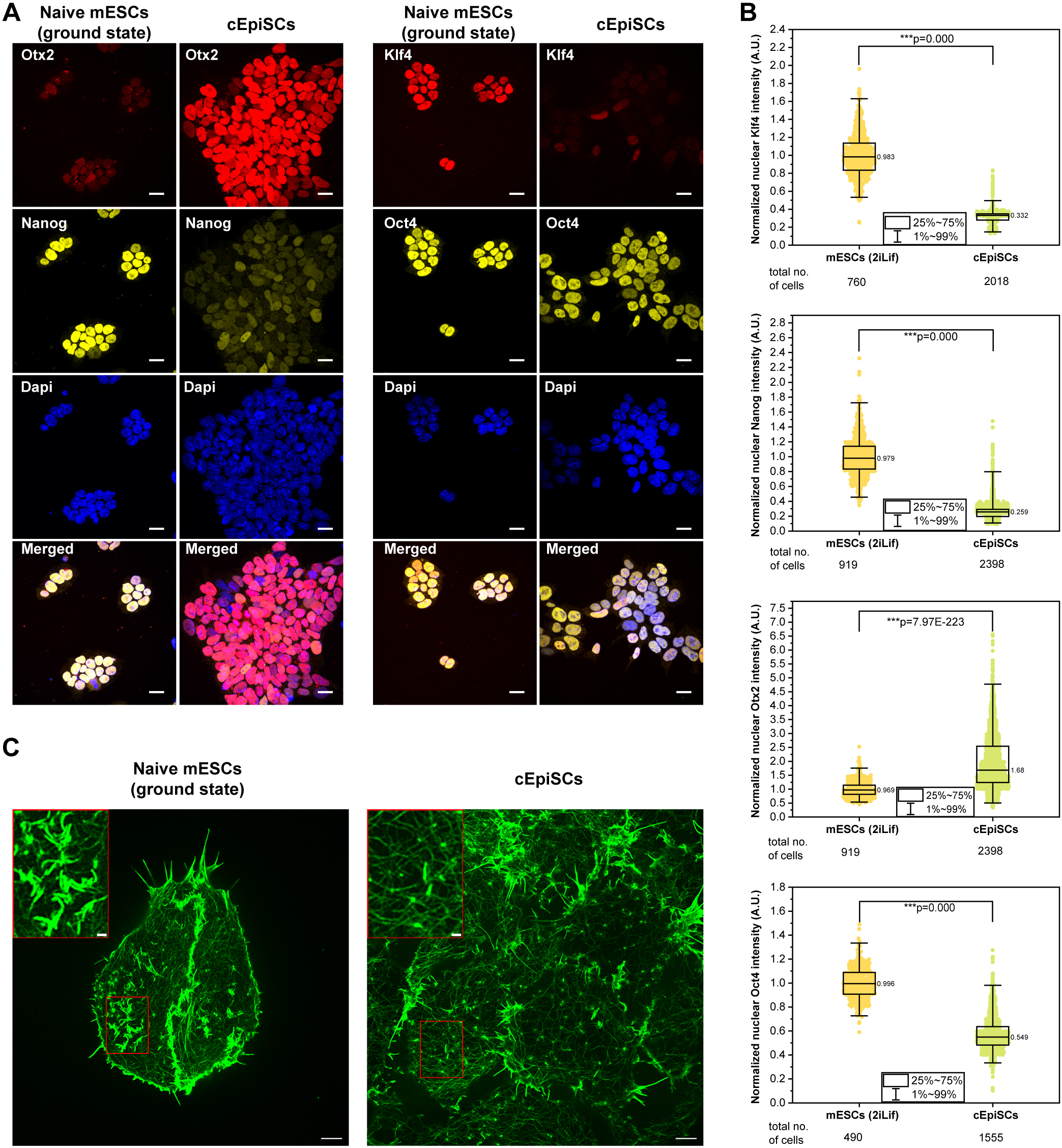
Ground-state PSCs are thought to resemble cells of the early embryo prior to implantation. To investigate how these cells maintain their naïve state, the researchers cultured mouse embryonic stem cells (mESCs) in 2iLIF medium, which stabilizes the ground state, and used super-resolution structured illumination microscopy to closely examine the actin cytoskeleton. They discovered previously undescribed, elongated actin-rich structures embedded in the cell cortex. These structures contained E-cadherin, α-catenin, β-catenin, and p120-catenin—components typically found at cell–cell junctions. However, unlike traditional junctions, these structures were not located at sites of cell contact and were also observed in isolated single cells.
The team named them non-junctional cadherin complexes (NJCCs). Further experiments revealed several unique properties of NJCCs. Laser ablation showed that these structures exist under very low mechanical tension, unlike conventional adherens junctions. Moreover, α-catenin remained in its “closed” (inactive) conformation, and calcium depletion, which typically disrupts cadherin-based adhesions, had no effect on NJCCs. These findings suggest that NJCCs form primarily via cis-interactions—where E-cadherin molecules within the same cell bind to each other—rather than trans-interactions that link adjacent cells.

Naïve ground-state mESCs exhibit unique actin-rich structures. Image from https://doi-org.libproxy1.nus.edu.sg/10.1242/jcs.263811
Using confocal microscopy, the team further confirmed that NJCC formation depends on the extracellular domain of E-cadherin, but not its cytoplasmic tail, which usually links cadherins to the actin cytoskeleton. This suggests that NJCCs may function as pre-assembled cadherin clusters, potentially primed to mediate rapid cell–cell interactions when required. Live-cell imaging showed that NJCCs are dynamic yet relatively stable, persisting for approximately 20 minutes before disassembling. When cells were induced to transition from the ground state to a more differentiated, “primed” pluripotent state using activin A and FGF2, NJCCs disappeared entirely—replaced by stress fibers and other cytoskeletal features characteristic of primed cells. This indicates that NJCCs could serve as a structural marker for distinguishing different pluripotent states.
To explore regulatory mechanisms, the team investigated the role of Rac1, a small GTPase known to control actin dynamics. Activation of Rac1 disrupted NJCCs and led to the dissociation of β-catenin. This was accompanied by a partial reduction in pluripotency gene expression, including key markers such as Nanog and Oct4, suggesting that NJCCs may play a role in maintaining the naïve state, with Rac1 acting as a molecular switch in this process.
While the exact role of p120-catenin and downstream signaling pathways remains to be fully elucidated, the findings point to specific Rac1-regulated pathways as key mediators linking adhesion, actin dynamics, and the unique architecture of ground-state stem cells.