Probing epithelial mechanics
Tools of the trade
Written by Sruthi Jagannathan | Illustration by MELANIE LEE | January 2019
The structural integrity of epithelial tissues and coordinated functioning of their constituent cells is important for several biological processes to take place normally. How epithelial tissues behave as active materials, and how such behavior is studied with the help of latest techniques that support their growth on highly customized, artificial substrates with mechanical properties that closely mimic physiological tissue environments as well as with tools that enable characterization of tissue response to a range of mechanical cues is summarized in this review co-authored by researchers from the Mechanobiology Institute and the Department of Biomedical Engineering, National University of Singapore, and Institut Jacques Monod, CNRS, France. The review is featured on the current issue of Nature Review Materials.
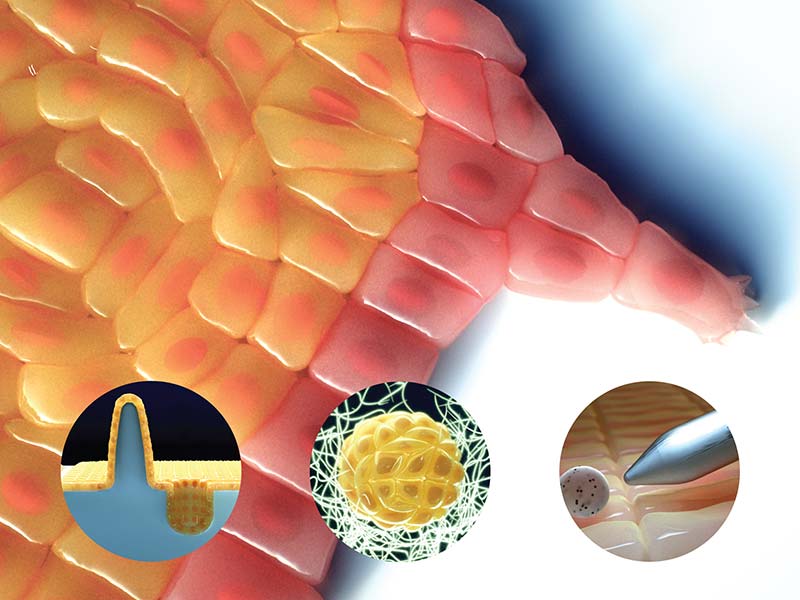
Tools that characterize nano-scale mechanics governing epithelial tissue mechanics
Epithelial tissue is one of the four major tissue types found in mammals and its intact organization and functioning is crucial for several fundamental biological processes – right from the growth and development of an organism during its fetal stages to the repair and regenerative processes that maintain homeostasis in adult life.
The diverse functions of the epithelial tissue can be attributed to its robust organization and highly-coordinated behavioral patterns, which need to be maintained amidst an extremely dynamic external environment. Such robustness arises from the constant receiving and relaying of vital cellular signals both between neighboring cells, and between cells and the supportive matrix (extracellular matrix or ECM) on which they reside. These signals are mainly biochemical (e.g. hormones, growth factors) or physical (mechanical properties such as shape and stiffness) and tissues rely on this information to quickly adapt to changes to their surroundings and stay intact to carry out their functions. Given the robustness of this biological system, it is not surprising that cellular events that lead to compromised tissue structure and functions form the basis for the onset and progression of several diseases, including cancers, inflammatory disorders, and so on. Owing to the direct impact it has on health and disease, an in-depth understanding of epithelial tissue behavior has attracted much attention amongst the scientific community in recent years.
Novel, state-of-the-art tools
The rapid advent of technology, coupled with increasing collaboration between biologists, physicists, material engineers, and scientists from other fields, has spurred research in the area of epithelial tissue mechanics. With novel, state-of-the-art tools now at their disposal, scientists are able to zoom into the nano-and micro-scale details of the molecular and cellular processes that drive the close crosstalk between biological systems such as epithelial tissues and their vibrant surroundings to gain insights into how this crosstalk is differently regulated in healthy and pathological conditions.
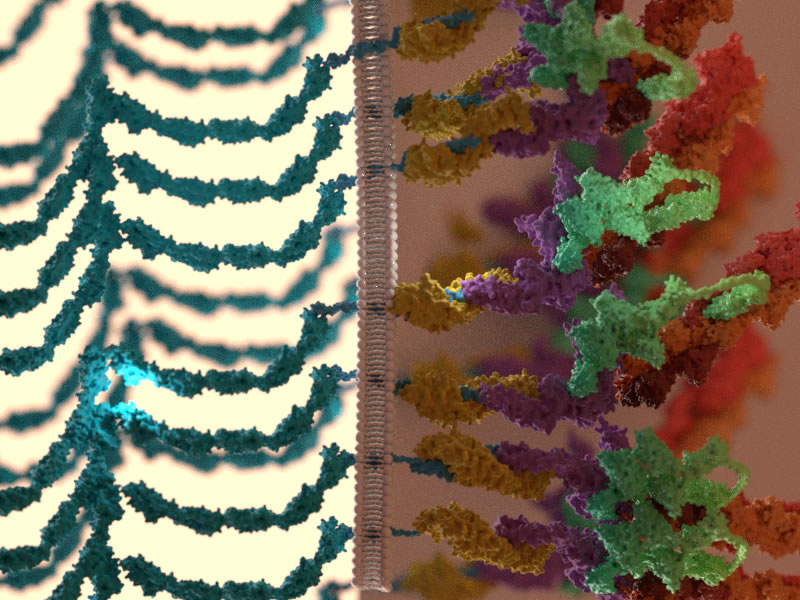
A recent review from the Mechanobiology Institute, National University of Singapore, published in Nature Reviews, throws light on the behavior of epithelial tissues as active materials that dynamically change their rheological properties (for example, their state of flow or deformation) in response to mechanical signals from their surroundings. It delves into the role of molecular assemblies called cell adhesions in mediating cellular communication with neighboring cells (via cell-cell adhesions) or with the ECM (via cell-matrix adhesions) and the significance of cellular communication in coordinating the collective behavior of cells, such as guiding their movement as a group. The precise regulation of cellular communication is essential for the coordinated movement of cells that gives rise to defined morphologies during embryo development or for gap closure during wound healing in adult organisms, but dysregulation in this communication can be responsible for the movement of small clusters of abnormal cells during cancer metastasis.
To be able to comprehend how tissue behavior is regulated by the constantly evolving three-dimensional surroundings, it is first essential to create controlled environments in the lab that closely mimic the dynamic, real-life ECM parameters.
To be able to comprehend how tissue behavior is regulated by the constantly evolving three-dimensional surroundings, it is first essential to create controlled environments in the lab that closely mimic the dynamic, real-life ECM parameters. A major section in this review effectively summarizes the latest tools and methodologies that can be used to engineer an impressively large array of 2D and 3D artificial substrates, and how they can be applied to optimize epithelial tissue growth and behavior. These substrates can be made of biopolymers such as collagen, or semi-synthetic or synthetic polymers such as polyacrylamide (PAA) or polydimethylsiloxane (PDMS), and a wide range of techniques are employed to precisely control one or more of their physical properties. These include altering epithelial geometry such as their free edges to control cell migration patterns, altering the biochemical composition and density on the substrate to control cell adhesion forces and modulate overall tissue dynamics, tuning the mechanical properties of the substrate such as stiffness and topography to study the various mechanosensing mechanisms at play, engineering 3D topographical features to closely mimic normal and disease physiology, and many more.
As well as creating controlled environments for optimizing tissue growth and behavior, it is also important to be able to quantify tissue responses to changes in their mechanical properties. Another section of the review outlines the latest suite of tools used by scientists to probe and measure the mechanical properties of epithelial tissues, in response to internal and external perturbations. While tools like atomic force microscopy (AFM), optical and magnetic tweezers aid in studying tissue behavior following mechanical perturbations, techniques such as forster resonance energy transfer (FRET) spectroscopy and laser ablation can provide precise estimates of endogenous force or tension levels existing between molecules, within subcellular structures, or across cells and tissues.
Novel, sophisticated tools that integrate principles in biology, physics, and material sciences have been central to recent developments in the field of epithelial tissue mechanics. With these tools able to replicate physiological conditions and predict real-time tissue responses as precisely as possible, scientists can now visualize and speculate the nano-scale mechanics that govern epithelial tissue mechanics in vivo, with a level of accuracy that was not possible before.
A detailed account of the range of 2D and 3D substrates used and the methodologies employed to probe epithelial mechanics can be found in the review.