Persistent α-Catenin Activation
Lakshmi Ramachandran | 10 October 2016
Members of the Groves Lab have discovered that α-catenin, once activated by forces applied during the process of E-cadherin clustering, remains activated irrespective of the presence of continued force. This new finding challenges the previous notion that activated α-catenin is force-sensitive. This study was led by Dr. Kabir H Biswas, a Senior Research Fellow at the Mechanobiology Institute (MBI), National University of Singapore, from the lab of Prof. Jay T. Groves, and published in the Biophysical Journal (Biswas KH et al., Sustained α-catenin activation at E-cadherin junctions in the absence of mechanical force, Biophys J. 2016 Sep 6;111(5):1044-52. doi: 10.1016/j.bpj.2016.06.027).
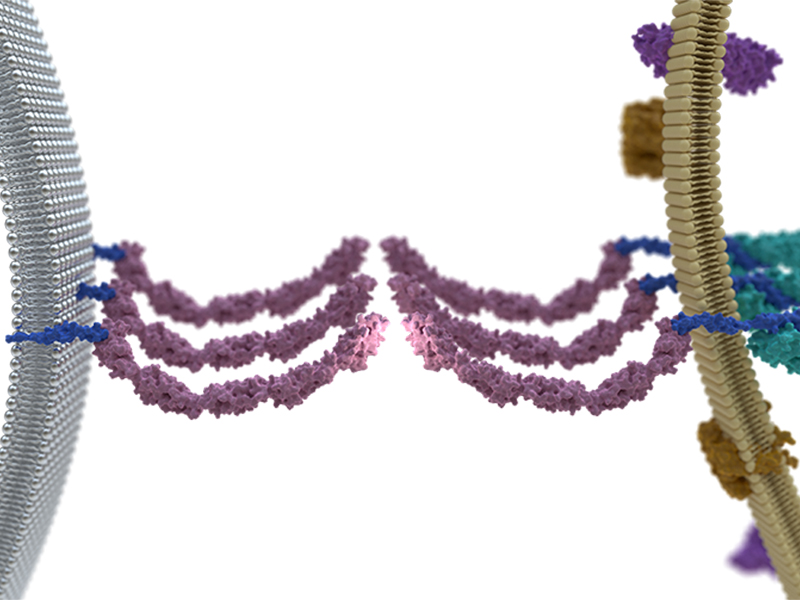
A schematic showing a cell making adhesive contacts with the supported lipid bilayer. Left inset shows peripheral E-cadherin clusters interacting with activated α-catenins and the actin cytoskeleton. Right inset shows central E-cadherin clusters with α-catenins, the majority of which are activated despite the absence of cytoskeletal contacts. FIGURE BY CHUN XI WONG
Switching behavior of α-catenin at e-caderin adhesions
Cells remain glued to each other in tissues through adhesive structures formed by dedicated proteins at cell-cell junctions. These Adherens Junctions (AJ) are highly dynamic, meaning that they can assemble and disassemble in response to various physical cues from the environment. The major component of AJs is E-cadherin, a protein that spans the cell membrane. The inner regions of E-cadherin interact with the structural proteins of the cell, mainly the actin cytoskeleton, while the outer regions connect to an E-cadherin on an adjacent cell.
The process of AJ formation in epithelial cells begins with the extension of finger-like exploratory projections, called filopodia, from the surface of adjacent cells. Protrusion and retraction of filopodia results in the formation of micro-clusters of E-cadherin at the cell-cell interface. Following this initiation of adhesion, additional proteins called catenins join in to link E-cadherin with the actin cytoskeleton, which strengthens and stabilizes the AJ.
Sticky proteins in cells
α-catenin is one of the three catenins found at AJs and is thought to function as a transducer of mechanical forces. Previous work has shown that α-catenin at E-cadherin adhesions undergoes force dependent activation from a ‘closed’ to an ‘open’ conformation. It is the open, activated form of α-catenin that binds actin. However, the precise mechanics of α-catenin activation was not known due to limitations in experimental systems. For example, monolayer cell cultures are often used to study cell-cell junctions, but in this live cell system it is difficult to control the dynamics of E-cadherin clusters or perturb E-cadherin cluster assembly.
α-Catenin chooses to remain activated upon activation by cellular forces
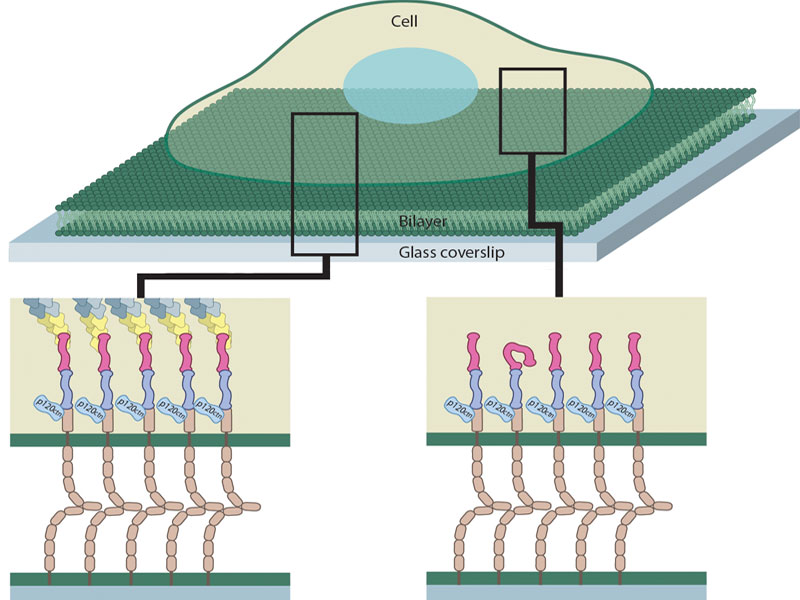
In order to overcome these limitations, Biswas et al. studied adhesion formation between a live cell and an artificial membrane functionalized with E-cadherin that mimics the cell membrane of the adjacent cell. This system is dubbed the hybrid live cell-supported lipid bilayer (SLB) platform and offers control over both the formation as well as inhibition of E-cadherin cluster assembly. E-cadherin cluster formation can be optimized on SLBs by controlling the density of E-cadherin and consequently their mobility, whereas E-cadherin assembly can be perturbed by using tiny, nano-scale grids on SLBs that physically block E-cadherin movement.
This study provides new insights into mechanical signal transduction at adherens junctions.
The scientists observed the interface between the cell and the SLB. E-cadherin clusters at the edge of the interface are known to be linked to the cytoskeleton, and consistent with this, activated α-catenin and actin were found to associate with these clusters. However, activated α-catenin additionally associated with E-cadherin clusters located in the centre of the interface, which were devoid of cytoskeletal proteins or force. Based on this the researchers noted that although E-cadherin cluster formation is essential for the initial activation of α-catenin, neither association with actin cytoskeleton, nor cellular forces, is required for the persistent activation of α-catenin.
This study provides new insights into mechanical signal transduction at adherens junctions. First, the study reveals a previously unknown mechanism of α-catenin activation, which is the requirement of E-cadherin clustering to initiate its activation. Second, the observation from this study that activated α-catenin is not sensitive to force, challenges the previous notion that α-catenin may function as a force-transducer at E-cadherin adhesions. This large pool of activated α-catenin that is not associated with actin filaments may be held in reserve by the cell in the event of an increase in intracellular tension.