Modelling nuclear dynamics
How fluctuations in the cell cortex influence nuclear shape
Andrew MS Wong, PhD. Illustration by Diego Pitta de Araujo, PhD | April 2018
By modelling the cell cortex as a gel, a collaborative team from the Mechanobiology Institute, Singapore and the Simons Centre for the Life Sciences, India has revealed how mechanical fluctuations regulate nuclear dynamics. Remarkably, this model explains how the same drug treatment can lead to drastically different effects on the nucleus depending on cell geometry and actomyosin contractility. This study was published in Physical Review Letters, the flagship publication of the American Physical Society.
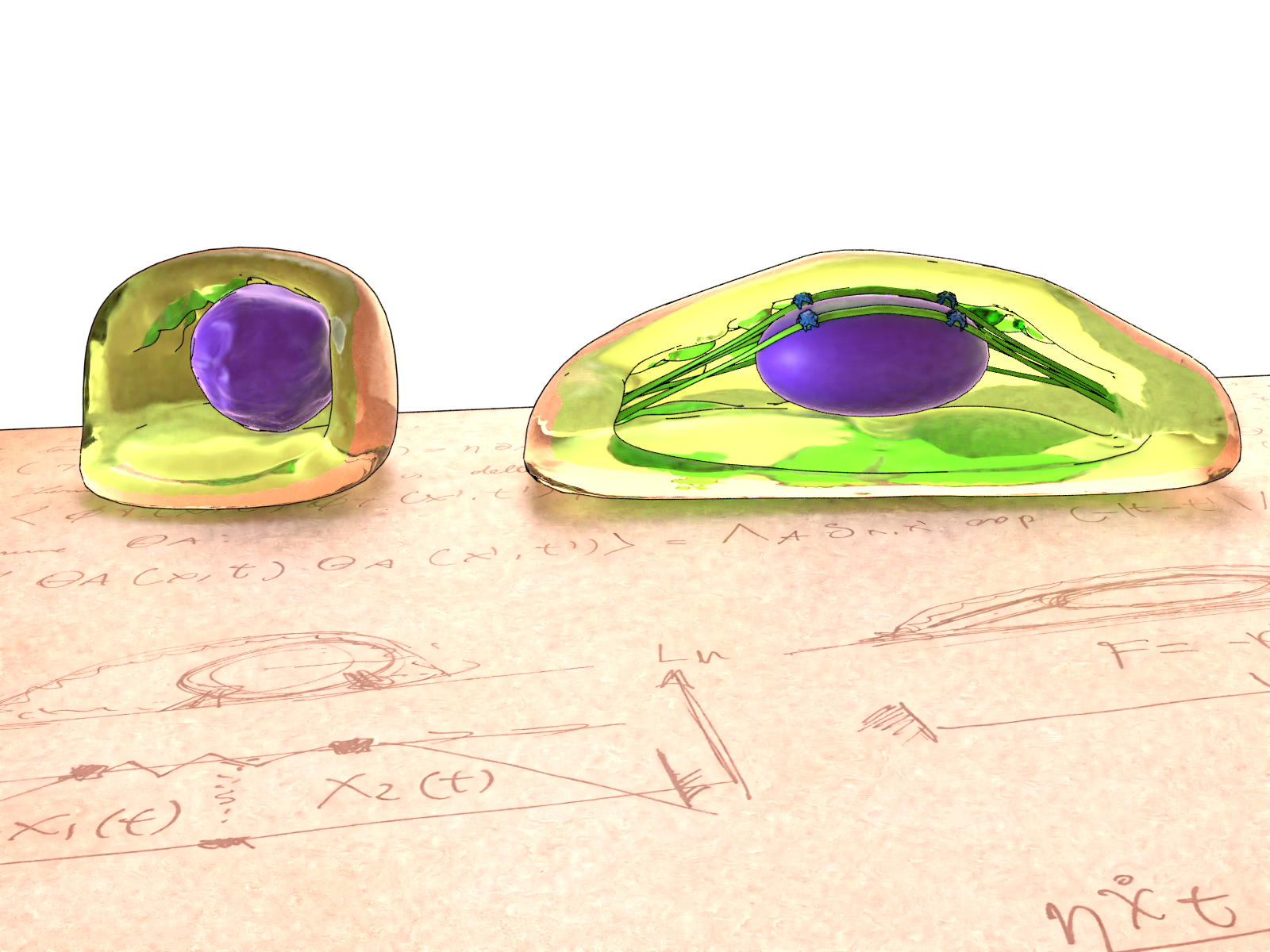
Artistic illustration of the cells grown on circular patterns (left) and rectangular patterns (right), and the theoretical models developed to investigate the behaviour of the cell cortex. Mature stress fibres in rectangular cells compress the nucleus, while the relaxed actin network in circular cells facilitate nuclear fluctuations.
Applying soft matter physics to understanding nuclear dynamics
Cells require energy to maintain their shape. The cell cortex – a meshwork of proteins that lines the inner part of the cell membrane – provides such energy through the contractile forces exerted by molecular motors over a network of actin filaments. A study published in 2016 (Makhija et al, doi: 10.1073/pnas.1513189113, featured in mbi.nus.edu.sg/science-features/shaping-cell-behavior) from the lab of Prof. G.V. Shivashankar at MBI have shown that the cell cortex fluctuations also control the shape of the cell nucleus.
They investigated the effects of cell geometric constraints and actin polymerization on nuclear dynamics. Cells were grown on rectangular patterns formed contractile actomyosin networks and mature stress fibers which restrain the nucleus and minimize nuclear fluctuations. Treatment with the actin depolymerizing drug Cytochalasin D disrupted the stress fibres and increased nuclear fluctuations, as visualized by a larger, more dynamic nucleus. In cells grown on circular patterns, actin filaments remained short and in flux, and the resultant actomyosin network could only generate a moderate level of contractile force, leading to a relatively unconstrained nucleus with increased fluctuations. However, further depolymerization of actin and reduction of actomyosin contractility with Cytochalasin D treatment resulted in an unexpected drop in nuclear fluctuations. Surprisingly, these experiments demonstrated that nucleus exists in its most dynamic and highly fluctuating state when actomyosin contractility was moderate, rather than at the extremes of low or high contractility.
The actomyosin cortex behaves as an active gel
In order to explain this, a collaborative effort was initiated between Prof. Shivashankar and the MBI Theory team headed by Prof. Jacques Prost together with theoreticians from the Simons Centre at NCBS Bangalore. Led by MBI research fellow Jean-Francois Rupprecht, the team started with the principle that molecular motor assemblies randomly agitate the cell cortex. When agitated at a short time scale (over seconds), the cell cortex behaves as an elastic solid. However, at larger time scales (over a few minutes), thermal energy fluctuations break the molecular structures that bind the actin filaments together, thus enabling the gel to flow. With the knowledge that the experimental studies were conducted over a long time span of about than 1 hour, the theoretical research team were able to model the cell cortex based on a hydrodynamic description called active gel theory.
Theoretical modelling reveals how mechanical fluctuations in the cell cortex regulate nuclear dynamics
Extending this model to investigate how fluctuations in the cell cortex affected nuclear dynamics, the scientists predicted that the local diffusion coefficient of the nucleus depends on its distance to the cell edges. They confirmed that the effect of thermal force fluctuations and the resistive viscous force balanced out, as expected from statistical physics arguments. However, the active mechanical noise fluctuations arising from the contractile actomyosin cortex, which are persistent over the timescale beyond which the cell cortex behaves as a fluid, may not be balanced by viscous forces. The researchers show that this imbalance leads to a generic fluctuation-induced accumulation of the nucleus in regions where the nucleus diffusion coefficient is low.
Cortical fluctuations regulate the shape of the nucleus
This role of actomyosin contractility in setting the intensity of the fluctuation-induced attraction on the nucleus explains the paradoxical experimental results observed in the experimental study. At a critical concentration of actomyosin, the nucleus is at its most dynamic. In rectangular cells, Cytochalasin D treatment shifts the actomyosin concentration towards this critical level, thereby increasing nuclear fluctuations. However, on a circular geometry the existing actomyosin concentration is already near this critical level, so further Cytochalasin D disruption now drives the concentration away, leading to a decrease in nuclear fluctuation.
The study shows that active fluctuations generate a specific fluctuation-induced attraction that cannot be described by just increasing an effective temperature of the cell. Interestingly, this model could affect the interpretation of a large class of particle tracking experiments. For example, it could be used in another biological situation to model how the dynamics of a tri-cellular inclusion are affected by the movement of the surrounding tissues. Furthermore, this study revealed how pharmacological drugs can have different biological effects depending on the cell geometry, reinforcing the important link between the 3D organization of the cell and function.











