Mechanics shaping the germline
Actomyosin corsets protect the epithelium
Written by Sruthi Jagannathan | Illustration by Diego Pitta de Araujo | January 2019
The maintenance of the syncytial architecture of the germline is essential for the growth and maturation of germline cells into functional gametes within the gonads. A recent study led by researchers from the Mechanobiology Institute, National University of Singapore, has identified a corset-like actomyosin structure lining the C. elegans syncytial germline, whose contractile activity generates tension important for balancing forces within the gonad and for maintaining the syncytial organization of the germline. This work is published in Nature Communications.
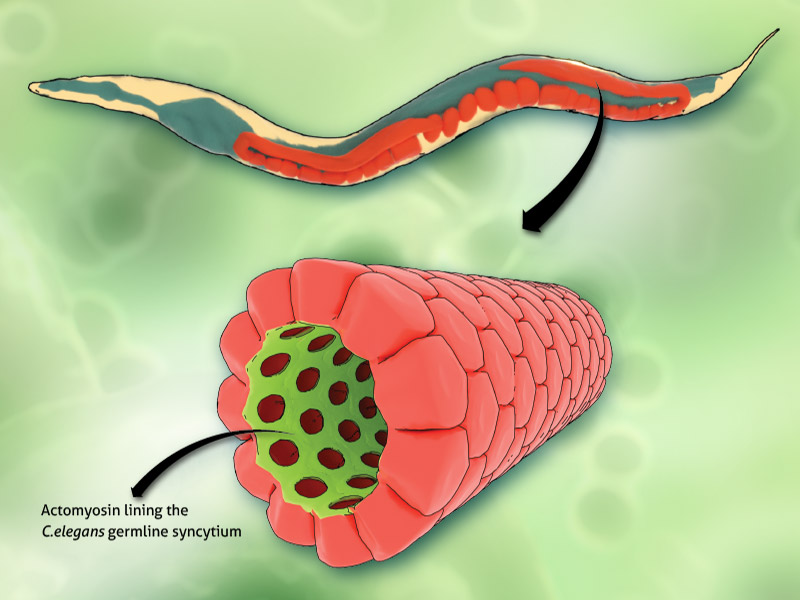
An artistic depiction of the corset-like actomyosin structure (in green) lining the syncytial germline within the C. elegans gonad.
A corset-like actomyosin structure that maintains the syncytial organization of the germline
The perpetuation of the human race, or any mammal for that matter, depends on the ability of its population to reproduce sexually and give rise to new offspring. To facilitate this foundational biological phenomenon, all sexually-reproducing animals possess specialized reproductive cells called gametes – sperms in the male and eggs in the female. The fusion of cytoplasmic and genomic content from the two gametes forms the basis for the creation of new life.
Gametes are formed within the reproductive organs of the body called gonads, by a process known as gametogenesis. In this highly conserved process, a series of cell types -constituting the germline- undergo sequential evolution into gametes. Any abnormality or deviation in this sequential process results in various fertility problems, ranging from under-production of gametes or production of non-functional gametes, to sterility.
In many animal species, right from insects to mammals, the germline cells are arranged into a unique tissue organization, known as a syncytium. Within the syncytium, hundreds of cells, which are partially surrounded by plasma membranes, share a common cytoplasmic pool. This unique and preserved tissue organization plays a critical role in supplying the germline cells with essential factors that are responsible for their growth and maturation into functional gametes.
Scientists have made great strides and clearly established the cascade of events that drive the reproductive process in detail – right from the genes and the corresponding proteins that come together to regulate the various steps to the tissue-level processes that eventuate in the fusion of gametes. However, the underlying mechanisms that help in maintaining the syncytial organization of the germline remain unclear. Given that the germline tissue, like any other body system, is subject to mechanical stress and strain arising from several factors within its surroundings, could mechanical cues arising from the tissue microenvironment be helping in keeping the syncytial organization of the germline intact?
How is the syncytial organization of the germline maintained?
Finding answers to this central question was the focus of a recent study led by a team of researchers from the Mechanobiology Institute, National University of Singapore that was led by Visiting Faculty Ronen Zaidel-Bar, who is also an Associate Professor at the Tel-Aviv University, Israel, along with Postdoctoral Fellow Dr Priti Agarwal. The research team carried out their studies in the nematode worn, Caenorhabditis elegans, which is both transparent and hermaphroditic, meaning that both male and female gametes are formed inside a single gonad within the same organism. This, together with the important characteristic that two-thirds of the C. elegans body is occupied by a large gonad, makes it an excellent model organisms for this study.
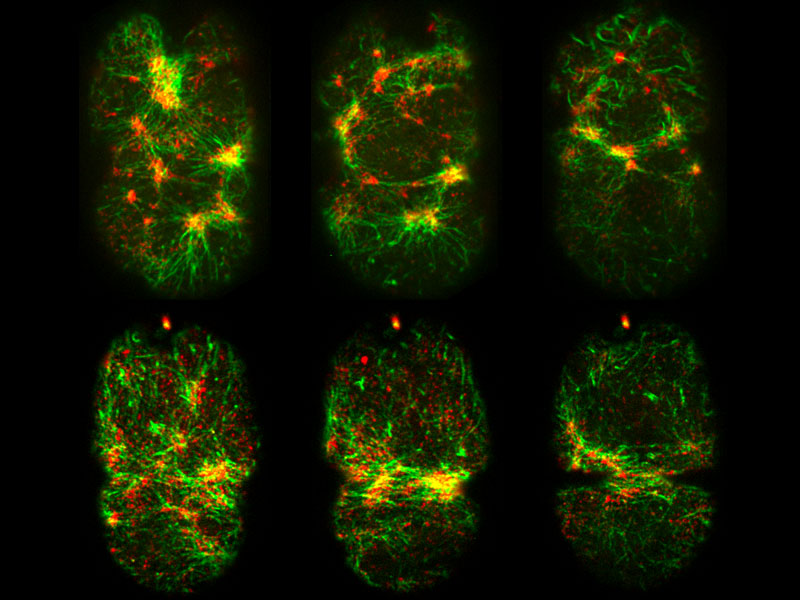
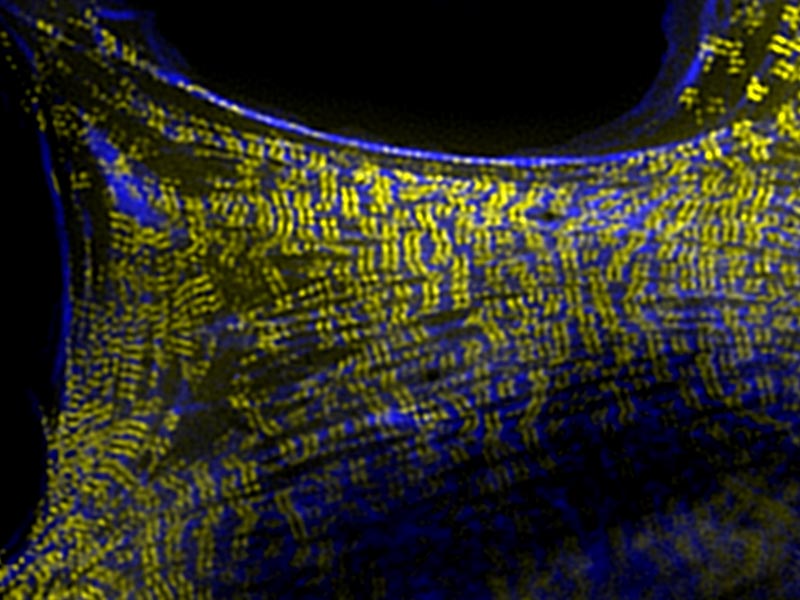
By using fluorescence confocal imaging techniques, Dr Agarwal and team identify the presence of specialized actomyosin assemblies forming corset-like structures around the central rachis in the C. elegans germline syncytium.
In C. elegans, the syncytial germline cells are connected to the shared cytoplasm, known as rachis, via openings at the cells’ apical (top) surfaces, also known as rachis bridges. Using fluorescence confocal imaging techniques, the researchers observed the presence of specialized molecular assemblies forming an inner corset-like structure around the central rachis.
Mainly comprised of filaments or bundles of actin and myosin proteins, these structures are well-known for their role as contractile machineries that generate forces required for movement in muscle cells or for other cellular processes in non-muscle cells. The researchers then used laser beams to make micron-sized incisions along several planes on the germline tissue to quantify the magnitude and direction of forces generated by these actomyosin structures, which is quantified by the recoil velocity of the surrounding tissue following the incision.
These experiments led the researchers to confirm the role of actomyosin structures as the predominant tension-generating structure in the germline tissue. Furthermore, they found that forces are generated along two directions: one along the plane of the rachis and the other perpendicular to the plane and these forces help in maintaining the appropriate height of the germ cells and the size of the rachis and rachis bridges amidst a mechanically dynamic tissue microenvironment – all of which is critical for maintaining the structural integrity as well as functionality of the germline.
This force balance helps in counter-balancing mechanical stress and strain caused by the continuous flow of cytoplasm from distal to the proximal end of the gonad required for oocyte growth and from the periodic contractions of sheath cells during ovulation. Based on their findings, the researchers proposed a corset-like function for the actomyosin structure lining the rachis that provides a supportive framework to maintain germline architecture.
The syncytial organization of the germline is evolutionarily conserved in multicellular organisms. By unraveling the pivotal role that the inner actomyosin structure lining the rachis plays in maintaining the germline organization in C. elegans, the study provides valuable insights into the mechanics that could possibly be governing germline organization in higher animals. This knowledge can provide scientists with a larger picture of the various underlying mechanisms that directly or indirectly control the correct functioning of the mammalian reproductive system, which could be responsible for variations in fertility.