Less is More: Simplifying Optimal 3D Interferometric Nanoscopy through Vortex Interference
The development simplifies a complex multi-channel interferometer to a single-channel system.
Researchers from the Kanchanawong Lab at MBI developed a new tool making cutting-edge 3D nanoscopy more accessible to laboratories worldwide.
For decades, scientists have pushed the limits of light microscopy to peer into the intricate machinery of life. Super-resolution microscopy techniques, which break the traditional diffraction limit, have revolutionized biology by allowing researchers to see individual molecules at work inside cells. But while pinpointing molecules in the horizontal plane (x-y) has become routine, achieving the same precision in the vertical (z) direction has remained a challenge—usually requiring complex optical systems with multiple detectors and complex calibrations.
A new study published in Physical Review Letters led by Wei Wang from the Kanchanawong Lab at the Mechanobiology Institute, National University of Singapore, introduces a solution that simplifies 3D super-resolution imaging while retaining the highest possible precision. The approach, called Vortex Interference Localization Microscopy (VILM), uses the wavefronts of light to encode depth information in a single image.
Traditional 3D single-molecule localization microscopy (SMLM) methods often rely on interferometry—splitting and recombining light waves to extract tiny differences in path length. The gold-standard 4Pi systems can localize molecules with near-quantum-limited precision, but they require three or four detection channels and specialized beam splitters, making them complex and expensive. Maintaining perfect interference across multiple channels also requires intricate precision, limiting widespread adoption of these techniques.
VILM sidesteps this complexity using vortex phase plates, optical elements that twist light into a “donut” shape often seen in STED or MINFLUX microscopy. When two donut-shaped beams with opposite orientations are combined in a 4Pi microscope, they interfere to produce a distinctive bilobed pattern that rotates as the fluorescent molecule moves up or down. This means that depth information can be read directly from the orientation of the pattern—no need for multiple phase-shifted images.
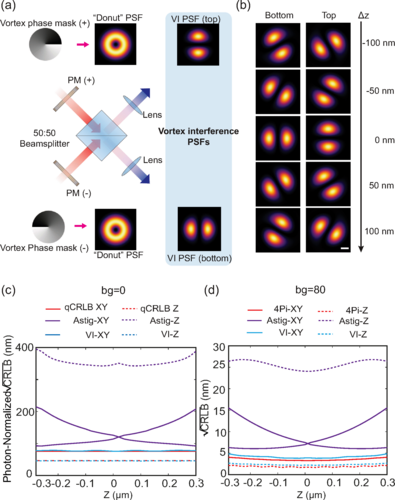
Numerical modeling showed that VILM achieves localization precision right at the theoretical quantum limit for a single beam-splitter interferometer. In practical tests, the researchers built a VILM setup using a simple 50:50 beam splitter and demonstrated nanometer-scale accuracy in 3D. The system achieved axial precision approximately two times the lateral precision, an ideal situation for volumetric imaging.
To put VILM to the test, the team imaged well-known cellular structures such as microtubules, which appeared with clear 3D profiles, showing their ~30 nm hollow diameter. The method was fast, requiring just minutes of setup and imaging, unlike previous interferometric systems that would have taken much longer to calibrate.
They also applied VILM to study integrin-based adhesions, the molecular structures cells use to anchor themselves to their surroundings. Using fluorescently labelled antibodies, the researchers mapped the precise 3D positions of key signalling proteins, finding them arranged in thin plaques just tens of nanometres above the surface—consistent with and extending previous high-resolution studies.
This development is as much about practicality as performance. By reducing a complex multi-channel interferometer to a single-channel system, VILM makes cutting-edge 3D nanoscopy more accessible to researchers without requiring elaborate optical engineering.
As super-resolution microscopy continues to uncover the nanometre-scale choreography of life, tools like VILM could help bring these discoveries to more labs worldwide—revealing cellular architecture with unprecedented clarity, one rotating “donut” at a time.