How Dying Cells Signal Growth: Mechanical Forces Drive Targeted Cell Proliferation after Apoptosis
Activation of YAP, a mechanotransducing protein involved in cell proliferation and death, depends on nuclear size and mechanical force propagation of cells.
Researchers from the Toyama Lab at MBI uncover how mechanical factors including force and nuclear size direct cell death and replacement. This study was published in Developmental Cell. https://doi.org/10.1038/s44318-024-00114-4
When cells naturally die by design, their fall sets the stage for a localized regenerative response known as compensatory proliferation – neighbouring cells undergo cell division to maintain tissue integrity and homeostasis. This phenomenon was first discovered over 40 years ago in Drosophila wing discs, which regrew to their original size even after 60% of their cells were destroyed.
Previous studies have shown that mitogenic signals – biochemical cues that initiate cell division – can trigger this regenerative response. Theoretically, these signals should uniformly trigger all neighbouring cells, however, only a few adjacent cells were found to be stimulated. This discrepancy has puzzled scientists and hinted that additional mechanisms could be spatially targeting cells for proliferation.
A multidisciplinary team of researchers from the Mechanobiology Institute (MBI) and the Department of Biological Sciences, National University of Singapore (NUS), in collaboration with partners from France and Japan has now uncovered a key player: mechanical forces. Their study suggests that the physical signals generated around a dying cell help spatially determine which neighbouring cells divide.
Time-lapse images of MDCK YAP-GFP monolayer stained with Hoechst 33,342 (magenta). Asterisk, apoptotic cell; white arrowhead, YAP-GFP nuclear translocation; scale bars, 20 μm. https://doi.org/10.1016/j.devcel.2023.01.005
Led by MBI research fellows Takumi Kawaue, Ivan Yow and graduate student Pan Yuping from the lab of Associate Professor Yusuke Toyama, the team discovered that the irregular pattern of compensatory proliferation corresponded to the non-uniform mechanosignals generated around the dying cell. To investigate this, they grew cells on a deformable substrate embedded with fluorescent beads. As a cell underwent apoptosis, it triggered a wave-like displacement of the beads – evidence of substrate deformation in response to mechanical forces.
The deformation was strongest near the dying cell and radiated outward, suggesting a release of tension in the tissue. This tension is generated due to the contraction of an actomyosin cable formed by the neighbouring cells to extrude the dying cell, and strong intercellular attachments that transmit mechanical stress. Together, these forces stretched nearby cells, creating a mechanically dynamic environment.
The team then looked at whether these mechanical signals are associated with known growth pathways. Interestingly, they found that only stretched cells near the dying cell activated YAP, a mechanotransducer – a protein that responds to mechanical signals by relocating from the cytoplasm of the cell into the nucleus, where it triggers the Hippo signaling chain.
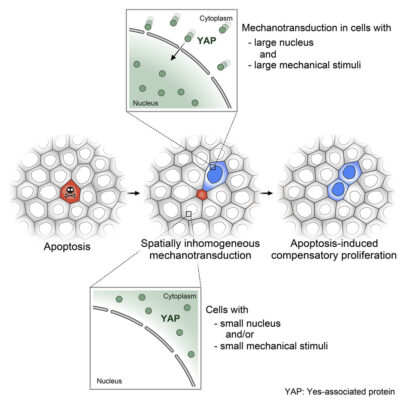
Graphical abstract illustration the role of mechanotransduction on apoptosis-induced compensatory proliferation. https://doi.org/10.1016/j.devcel.2023.01.005
Further investigation revealed a surprising layer of specificity: only cells with a larger nucleus were responsive to YAP activation and subsequent proliferation. More importantly, having a large nucleus alone was not sufficient, as cells also had to be mechanically stretched. This was discovered through disruption of mechanical force propagation by either shrinking the tissue or increasing the attachment strength of the substrate to the underlying surface, resulting in significant reduction of YAP activation and cell division. This confirms the crucial role of mechanical signals in determining tissue behaviour.
Ivan Yow, Research Fellow: At the beginning, we observed the mechanical waves which fascinated us. However, frustration weighed heavily on me as I could not interpret this fascinating phenomenon and started doubting myself if this was all a mistake or caveat. It took years before I realized that what I thought was a mistake could be the doorway to a discovery I never imagined.
Apoptosis is an intricately-regulated process that ensures just the right amount of cell proliferation can sufficiently replace the dead cells without overpopulating the tissue. While there still remains much unknown about the phenomenon of compensatory proliferation in maintaining homeostasis, the MBI team have demonstrated that solving this biological mystery will require a fresh perspective that incorporates the role of mechanical force.