DECIPHERing the Role of Cell-Matrix Interactions in Ageing Heart Health
A biomimetic hydrogel-hybrid system enables tunability of ECM properties to elucidate mechanisms underlying fibroblast activation and ageing.
Researchers from the Soft Nano-Biomaterials Lab at MBI developed a material system to enable precise investigation into how individual ECM properties affect cultured heart cells.
In a recent study published in Nature Materials, researchers from the Mechanobiology Institute (MBI) and Department of Biomedical Engineering, National University of Singapore (NUS) have developed a material system called DECIPHER (DECellularized In Situ Polyacrylamide Hydrogel-ECM hybRid). This biomimetic platform replicates the extracellular matrix (ECM) surrounding heart cells and enables precise investigation into how individual ECM properties affect cultured heart cells.
Led by PhD student Avery Rui Sun from the lab of Assistant Professor Jennifer Young, the team used DECIPHER to discern the individual roles of two ECM properties – ligands and stiffness – in influencing cardiac fibroblast behaviour in an age-dependent manner. As fibroblasts play crucial roles in heart remodelling during ageing and disease, these insights could have important implications for the development of cardiac rejuvenation therapies.

Asst Prof Jennifer Young (right) and PhD student Avery Rui Sun (left), who are from the College of Design and Engineering at NUS, image heart cells (orange) on DECIPHER scaffolds (pink) using a confocal microscope to understand how cells interact with the extracellular matrix.
Cells in our body are in constant communication with their surrounding ECM – a complex network of structural and biochemical molecules that direct essential cellular functions, including movement, division, and differentiation. In the heart, the ECM is highly dynamic; its properties, including ligand composition, structural organization, and mechanics, constantly change in response to physiological changes, including age. One notable change is increased stiffness, partly driven by changes in the ECM.
Cardiac fibroblasts are central to regulating the heart’s ECM. In healthy young hearts, they remain in a resting (quiescent) state. However, in response to external stresses, they become activated and differentiate into myofibroblasts, contributing to a dense and stiff matrix through ECM secretion and remodelling. This process is known as fibrosis, the cause of many cardiovascular disorders.
Although previous engineered biomaterial systems have revealed matrix-based mechanisms of fibroblast activation, it remains a challenge to independently control specific ECM properties. Adjusting one parameter, such as stiffness, can unintentionally alter others like ligand organization, making it difficult to determine which property actually contributes to cardiac fibroblast function. Compending the issue, many systems do not preserve the native architecture of the heart’s ECM.
To overcome these limitations, the team developed DECIPHER – a hydrogel-ECM hybrid scaffold that combines synthetic hydrogels with decellularized cardiac ECM. To achieve this, they obtained cardiac tissues from young or aged mice and stabilized them using a polyacrylamide hydrogel mesh with tunable stiffness, simulating the soft environment of a young heart or the stiffness of an aged one. The tissue was then decellularized, removing cells while leaving the ECM architecture and biochemical composition intact. This process produced four types of DECIPHER samples: young or aged ECM embedded in soft or stiff hydrogels, effectively decoupling ligand composition from stiffness.
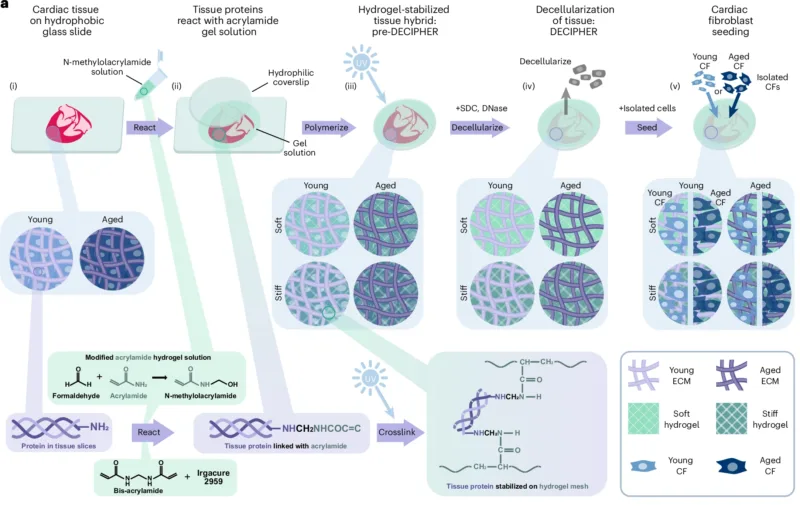
The DECIPHER system at a glance. Image credit: https://www.nature.com/articles/s41563-025-02234-6
The researchers then seeded the samples with cardiac fibroblasts from young and aged mice to study the individual effects of ECM ligands versus stiffness on cell function. Analysis of gene and protein expression showed clear distinctions in cell behaviour depending on both the age of the fibroblasts and the properties of the samples. Aged cardiac fibroblasts cultured on stiff, aged ECM exhibited increased markers of fibroblast activation and cellular ageing, whereas young fibroblasts on soft, young ECM samples were largely in a quiescent state, similar to what is seen in native tissues.
Interestingly, the team also found that these cellular states could be reversed by altering ECM ligand and stiffness cues with DECIPHER. Young fibroblasts grown on stiff, aged ECM samples eventually adopted an activated and aged state, while aged cardiac fibroblasts cultured on young ECM samples – regardless of stiffness – showed signs of rejuvenation. These findings suggest that aged fibroblasts have a reduced sensitivity to mechanical cues, with ligand composition playing a more dominant role in dictating their behaviour.

The DECIPHER sample consists of heart tissue (centre) embedded within a stiffness-tuneable hydrogel.
“This showed us that of the two ECM cues we controlled with DECIPHER, the ligands around aged cells matters more than the stiffness,” said PhD student Avery Rui Sun. “For young cells, however, both of these cues–aged ECM or aged stiffness–can cause them to prematurely ‘age’ and exhibit dysfunctional behaviour.”
The DECIPHER system represents a robust and tunable biomimetic system for understanding the dynamics of cell-ECM interactions and the distinct roles of individual ECM properties in determining cell state and function. By highlighting the key role of ECM ligands in age-dependent activation of cardiac fibroblasts, the study reveals specific matrix targets that could be targeted in the development of therapies for age-related heart disease. Moreover, the platform’s versatility means it could be applied to a wide range of tissue types to uncover ECM-driven mechanisms of dysfunction in ageing or disease.
“Many age-related diseases involve complex changes in ECM—not just in the heart,” said Asst Prof Young. “This means that we can use DECIPHER to study other disorders and uncover how cells interpret these complex matrix cues.”
The study was a collaboration involving researchers from The College of Design and Engineering – Biomedical Engineering Department, MBI, Department of Biological Sciences at the NUS Faculty of Science, NUS Yong Loo Lin School of Medicine, and the Cardiovascular Research Institute at the National University Heart Centre Singapore.






