A molecular brake
How BNIP-2 scaffolds GEF-H1 and RhoA to control cell migration
Written by Sruthi Jagannathan | Schematic provided by the Low Lab, edited by Diego Pitta de Araujo | September 2020
The regulation of cell migration is essential to prevent disease states such as cancers. In this study, Research Fellows Pan Meng and Ti Weng Chew, along with Principal Investigator Associate Professor Low Boon Chuan at the Mechanobiology Institute (MBI), National University of Singapore (NUS) reveal a role for BNIP-2 protein in scaffolding GEH-H1 and RhoA, following microtubule disassembly, and describe how this scaffolding is important for RhoA-dependent regulation of cell migration. The study is published in Science Advances.
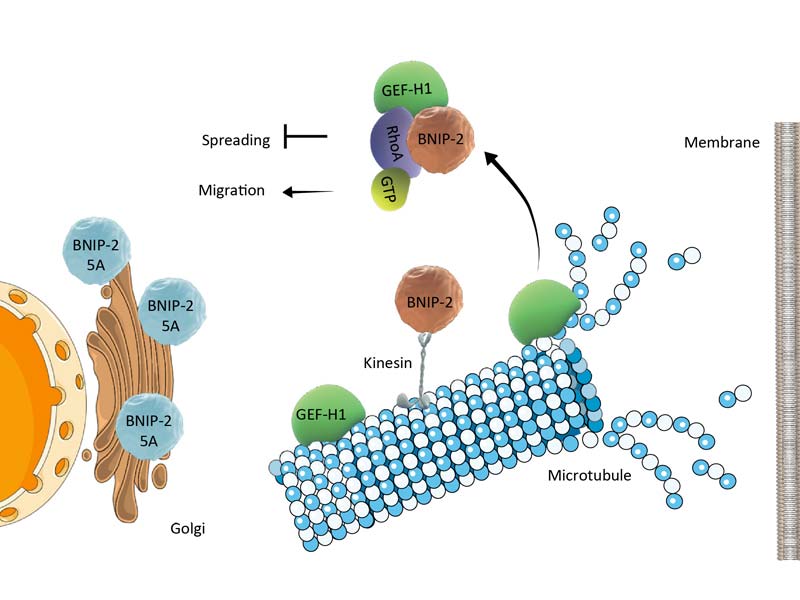
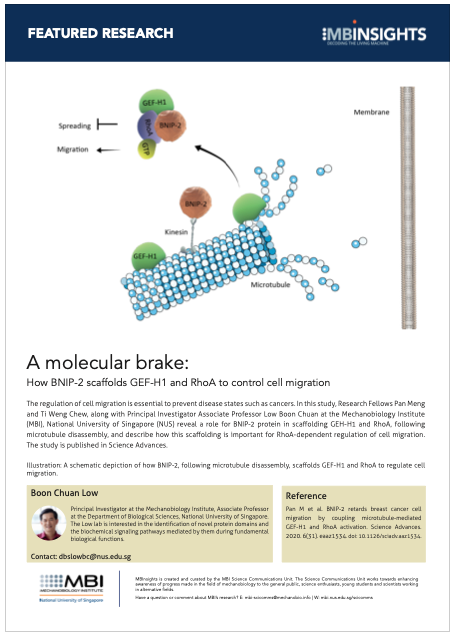
A schematic depiction of how BNIP-2, following microtubule disassembly, scaffolds GEF-H1 and RhoA to regulate cell migration.
BNIP-2 physically couples GEF-H1 and RhoA to regulate crosstalk between microtubule dynamics and cell migration
‘We got introduced through a mutual friend at a party’– many of us probably have such fond memories over how we met our best buddies.
Something similar happens in the microscopic world of proteins inside the cells of our body, as well. Two proteins, with a specific end goal (known as a cellular function, such as cell growth, or cell division) are brought together physically in space and time by a third protein that can bind to both of these proteins. This third ‘mutual partner’ protein is called a scaffold protein and it has to be present in optimal amounts to carry out its scaffolding functions most effectively.
The present study unravels the role of one such scaffold, BNIP-2, in physically coupling two proteins -RhoA and GEF-H1 (see illustration). RhoA is a small GTPase – a family of proteins that mediate important biochemical signaling pathways by switching between active GTP-bound and inactive GDP-bound states. Specifically, RhoA controls cell migration within tissues, and low RhoA activity in many cell types is associated with increased cell migration and cancerous properties of cells.
By identifying the role of the BNIP-2 scaffold in RhoA-dependent regulation of cell migration and how this is altered in some cancers, the present study creates possibilities for the development of interventions that specifically target such scaffolds and enable better treatment and management of the disease.
GEF-H1 is essential for maintaining RhoA in an active GTP-bound state. However, for GEF-H1 to activate RhoA, it has to be first released from microtubules (the cytoskeletal component to which it is initially bound) following microtubule disassembly. Therefore, microtubule dynamics has a major role in regulating GEF-1-RhoA coupling, RhoA activation, and downstream cellular functions.
BCH domain in BNIP-2 scaffolds GEF-H1 and RhoA
How this coupling actually happens in cells has remained a central question open to investigation. Researchers from the Mechanobiology Institute, National University of Singapore, led by Research Fellow Pan Meng, Research Fellow Ti Weng Chew, and Principal Investigator Boon Chuan Low, have tried to understand the scaffolding mechanism and the spatial regulation of BNIP-2 as the basis for addressing this question.
By employing a combination of protein-protein interaction and live cell imaging assays, they revealed important molecular details about how BNIP-2 scaffolds RhoA and GEF-H1, and how this influences cell migration. The key findings from their study were that BNIP-2 is vital for GEF-H1 coupling to RhoA, and the scaffolding effects of BNIP-2 are mediated by a specific region known as the BCH domain that serves as an attachment site for both RhoA and GEF-H1.
Accordingly, when BNIP-2 was depleted from cells, RhoA activity was suppressed, as GEF-H1 could no longer couple RhoA in the absence of the scaffold. At the cellular level, this reduced RhoA activity was associated with increased cell migration.
The researchers further noted that BNIP-2 binds to kinesin – a motor protein found on microtubules – and that this binding is essential for BNIP-2 scaffolding functions, confirming the role of the crosstalk between microtubule dynamics and cell migration.
Much like the mutual friend who makes vital connections between people, scaffold proteins can have an integral role in maintaining functional protein networks. From this standpoint, the findings described in this study highlight the important role of BNIP-2 in scaffolding RhoA and GEF-H1 and how this is important for controlling cell migration.
Enhanced cell migration, both as single and collective cell behaviour, is a hallmark of cancer cells. The identification of the BNIP-2 scaffold, and similar molecular scaffolds in future studies, will not only add to the current understanding of disease pathogenesis, but also create possibilities for the development of interventions that specifically target such scaffolds – paving way for better treatment and management of several cancer types.