What is the CLIC/GEEC Endocytosis pathway?
The CLIC/GEEC (CG) pathway is a clathrin-independent endocytic pathway mediated by uncoated tubulovesicular primary carriers called clathrin-independent carriers (CLICs) which arise directly from the plasma membrane and later mature into tubular early endocytic compartments called Glycosylphosphotidylinositol- anchored protein (GPI-AP) enriched compartments (GEECs). The GEECs then fuse with sorting endosomes in a Rab5 and PI3K dependent manner [1].
This endocytic pathway was first identified for its selective internalization of various GPI-APs like the folate receptor, in a manner independent of clathrin, caveolae and dynamin, and regulated by the Rho family GTPase Cdc42 [2]. Apart from endocytosis of GPI-APs, the CG pathway also facilitates the endocytosis of transmembrane proteins like CD44, dysferlin (reviewed in [3]) as well as entry of cholera toxin binding subunit, the vacuolating toxins aerolysin and VacA, and bulk fluid and membrane [4], [5], [6].
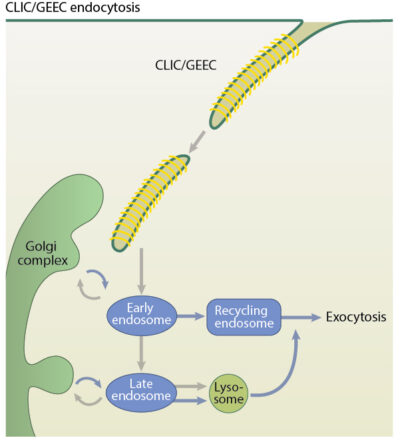
The clathrin-independent carriers (CLIC)/ Glycosylphosphotidylinositol- anchored protein (GPI-AP) enriched compartments (GEEC) endocytosis pathway
Role of CG pathway in membrane turnover
The CG pathway is a pinocytic pathway that accounts for the major uptake of fluid and bulk membrane in fibroblasts and is therefore a major contributor to membrane dynamics. About 40% of the endocytosed fluid is regurgitated within 5 minutes, quickly returning significant portions of the internalized plasma membrane and enabling rapid membrane turnover for key cellular processes like plasma membrane repair and homeostasis [6]. In mouse embryonic fibroblasts, the entire membrane area is recycled in less than 15 minutes via the CG pathway [4]. The CG pathway may play a role in repair of plasma membrane lesions induced by bacterial toxins by rapidly removing the toxin from the membrane through endocytosis. This is observed in the case of bacterial streptolysin O toxin (SLO) induced PM lesions. Also, dysferlin, a protein that regulates membrane fusion for muscle repair is found to associate with CLICs, further pointing to a role for the CG pathway in membrane repair (reviewed in [7]).
Regulation of CLIC/GEEC pathway
At the plasma membrane, GPI-APs are organized into cholesterol-dependent nanoscale clusters which are formed by the activity of cortical actin [8]. Indeed, cholesterol sensitive Cdc42-based recruitment of actin polymerization machinery is critical for the CG pathway [9].
Cdc42 dynamics at the plasma membrane, cycling between active (GTP-bound) and inactive (GDP-bound) states, is required for the recruitment of actin polymerization machinery in the CG pathway. Regulation of Cdc42 dynamics is initiated by GBF1, an Arf1 guanine nucleotide exchange factor (GEF) that activates Arf1 [10] [11]]. The activated Arf1 protein recruits a Rho GTPase activating protein (ARHGAP10), which inactivates Cdc42 and returns Cdc42 to its cycling state. Another regulator of Cdc42 and the CLIC/GEEC pathway is the GTPase Regulator Associated with Focal Adhesion Kinase-1 (GRAF1), which contains a RhoGAP domain that can inactivate Cdc42, a BAR domain and an SH3 domain [12]. Although dynamin is not required for vesicle budding in CLIC/GEEC pathway, it is seen to associate with the vesicles, post internalization (reviewed in [13]).
References
- Kalia M, Kumari S, Chadda R, Hill MM, Parton RG, and Mayor S. Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3′-kinase-dependent machinery. Mol. Biol. Cell 2006; 17(8):3689-704. [PMID: 16760436]
- Sabharanjak S, Sharma P, Parton RG, and Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2002; 2(4):411-23. [PMID: 11970892]
- Mayor S, Parton RG, and Donaldson JG. Clathrin-independent pathways of endocytosis. Cold Spring Harb Perspect Biol 2014; 6(6). [PMID: 24890511]
- Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, Pagano RE, Hancock JF, Mayor S, and Parton RG. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J. Cell Biol. 2005; 168(3):465-76. [PMID: 15668297]
- Gauthier NC, Monzo P, Kaddai V, Doye A, Ricci V, and Boquet P. Helicobacter pylori VacA cytotoxin: a probe for a clathrin-independent and Cdc42-dependent pinocytic pathway routed to late endosomes. Mol. Biol. Cell 2005; 16(10):4852-66. [PMID: 16055501]
- Howes MT, Kirkham M, Riches J, Cortese K, Walser PJ, Simpson F, Hill MM, Jones A, Lundmark R, Lindsay MR, Hernandez-Deviez DJ, Hadzic G, McCluskey A, Bashir R, Liu L, Pilch P, McMahon H, Robinson PJ, Hancock JF, Mayor S, and Parton RG. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 2010; 190(4):675-91. [PMID: 20713605]
- Howes MT, Mayor S, and Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr. Opin. Cell Biol. 2010; 22(4):519-27. [PMID: 20439156]
- Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, Chadda R, Vishwakarma R, Rao M, and Mayor S. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell 2008; 135(6):1085-97. [PMID: 19070578]
- Chadda R, Howes MT, Plowman SJ, Hancock JF, Parton RG, and Mayor S. Cholesterol-sensitive Cdc42 activation regulates actin polymerization for endocytosis via the GEEC pathway. Traffic 2007; 8(6):702-17. [PMID: 17461795]
- Kumari S, and Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat. Cell Biol. 2007; 10(1):30-41. [PMID: 18084285]
- Gupta GD, Swetha MG, Kumari S, Lakshminarayan R, Dey G, and Mayor S. Analysis of endocytic pathways in Drosophila cells reveals a conserved role for GBF1 in internalization via GEECs. PLoS ONE 2009; 4(8):e6768. [PMID: 19707569]
- Lundmark R, Doherty GJ, Howes MT, Cortese K, Vallis Y, Parton RG, and McMahon HT. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr. Biol. 2008; 18(22):1802-8. [PMID: 19036340]
- Kumari S, Mg S, and Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010; 20(3):256-75. [PMID: 20125123]


