What factors regulate actin filament polymerization?
Nucleation Promoting Factors (NPFs) (e.g. WASP, Scar/WAVE) modulate actin filament nucleation by bringing together actin monomers and pre-existing actin filaments, for example, during filopodial initiation where they recruit the Arp2/3 complex. NPFs compete with profilin for binding to free actin (which inhibits actin nucleotide exchange) [1][2][3][4]; these combined functions promote actin-filament assembly at the barbed end.
Mammalian NPFs are broadly grouped into 2 classes:
Class I proteins are classified into five groups:
1) Wiskott-Aldrich Syndrome protein (WASP) and neuronal-enriched homologue of WASP (N-WASP)
2) WASP family Verprolin-homologous (WAVE) proteins (aka Suppressor of cAMP receptor [Scar])
3) WASP and Scar homologue (WASH)
4) WASP homologue associated with actin, membranes and microtubules (WHAMM)
5) junction-mediating regulatory protein (JMY)
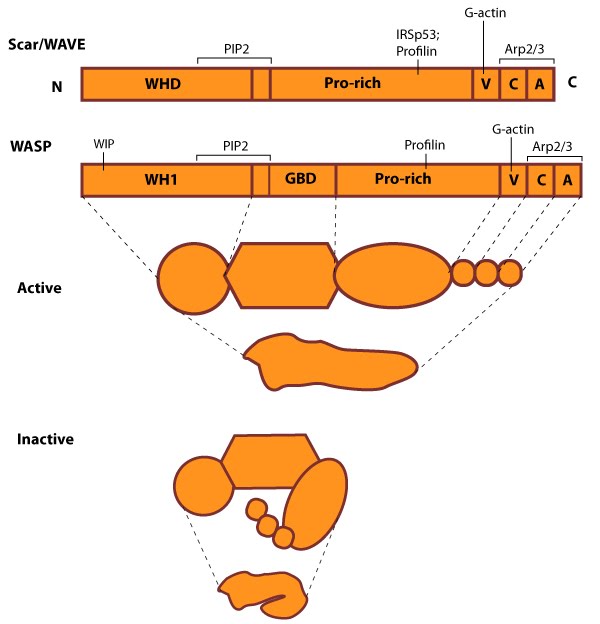
The molecular organization of Scar/WAVE family members and WASP, with examples of how the NPFs are represented in figures throughout this resource. Relevant domains believed to be important for binding to actin and for protein-protein interactions are highlighted (reviewed in [2, 13]). WHD= WAVE (also known as SCAR)-homology domain; WIP= WASP-interacting protein; V= verprolin-homology domain (i.e. WASP-homology-2 domain [WH2]); C= cofilin-homology domain; A= acidic-rich domain; WH1= WASP-homology-1 (aka Ena-VASP-homology-1 [EVH1]); GBD= GTPase binding domain. Of note: N-WASP contains an additional V domain, and the Scar/WAVE proteins lack a direct binding domain for the Rho GTPase family.
The conserved verpolin-cofilin-homology and acidic-rich (VCA) domain at the carboxy terminus of WASP and Scar family members binds directly to the Arp2/3 complex to increase its nucleation activity [5][6][7][8]. WASp also associates with other signaling components (e.g. haemopoietic cell kinase) and formins to modulate actin polymerization for cell polarization and chemotaxis in neutrophils [9][10]. WASp and formins also cooperate to control the balance between lamellipodial protrusion activity in epithelial cells [11]. The WASP family of NPFs are normally auto-inhibited due to protein interactions which prevent the NPF from associating with actin and the Arp2/3 complex; they are activated by *Rho GTPases and PIP2 [6] (reviewed in [12][13]). In contrast, the Scar/WAVE proteins have constitutive activity [14]. NPF accessory proteins also modulate the activity of NPFs.
NPF Accessory Proteins
Examples of NPF accessory proteins include Verprolin (yeast), which modulates the activity of WASp with type I myosins, to promote actin assembly by Arp2/3 complex [15]. WASp-interacting proteins (WIPs) will also regulate the WASp activity. For example WIP [16] not only inhibits N-WASP, but also promotes nucleation and activation of the [Arp2/3 complex through the coordinated binding of actin and another NPF, cortactin [17]. SPIN90/WISH (SH3 protein interacting with Nck, 90 kDa/WASP-interacting SH3 protein) [18]) on the other hand increases actin assembly in dendritic filopodia/spines independently of N-WASP through its association with the neuron-specific scaffolding protein, PSD-95[18]. Surprisingly, indirect evidence shows that WIPs are required for WASp function [19].
In another example, the WAVE complex will inhibit Scar/WAVE proteins from activating the Arp2/3 complex. The NPF activity of Scar/WAVE is restored during nucleation when Rac-GTP causes the dissociation of the WAVE complex from WAVE1 [20]. Similarly, IRSp53 has been implicated in both lamellipodia and filopodia formation/protrusion by augmenting the Rac-GTP-induced activation of WAVE NPF activity [21][22].
Ena/VASP
Proteins of the Ena/VASP family contribute to cell movement, axon guidance, neural tube closure and shape change in vertebrate cells by modulating actin filament organization and dynamics; these effects are achieved in part by regulating the morphology and behavior of actin-based structures such as lamellipodia and filopodia (reviewed in [23]). Ena/VASP proteins also modulate actin dynamics at sites of cell-ECM and cell-cell interactions and they are concentrated to the proximal portion of phosphotyrosine-rich domains at the ends of F-actin stress fibers [24].
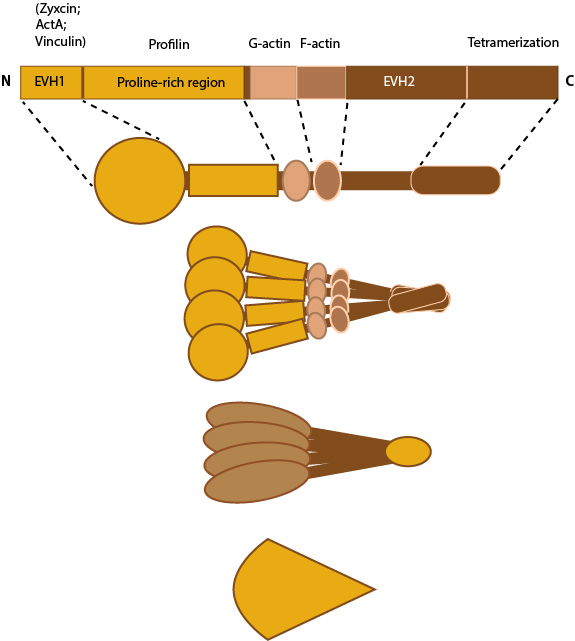
This schematic diagram illustrates the molecular organization of Ena/VASP.
Ena/VASP proteins promote actin filament elongation by tethering actin filaments to sites of active actin assembly [25][26][27]. Ena/VASP proteins recruit actin nucleation and initiation factors (e.g. Arp2/3 complex, formins) and promote F-actin assembly through profilin-binding (reviewed in [28]). The rate of Ena/VASP assisted actin filament elongation is determined by the recruitment of G-actin. This will occur via a G-actin binding site (GAB) that lies within the EVH2 domain and shares close sequence homology to WASP homology 2 motifs [29]. Ena/VASP proteins are also thought to accumulate at the plasma membrane where they alter actin polymerization by antagonizing the barbed (+) end capping proteins, thereby enabling the incorporation of actin into longer filaments [25][26]; however, controversy over their exact mechanism still exists (reviewed in [23]). In addition, Ena/VASP may promote actin assembly by an unknown mechanism that is independent of initiation factors, however, this has not been demonstrated in intact cells [30].
References
- Chereau D, Kerff F, Graceffa P, Grabarek Z, Langsetmo K, and Dominguez R. Actin-bound structures of Wiskott-Aldrich syndrome protein (WASP)-homology domain 2 and the implications for filament assembly. Proc. Natl. Acad. Sci. U.S.A. 2005; 102(46):16644-9. [PMID: 16275905]
- Suetsugu S, Miki H, and Takenawa T. The essential role of profilin in the assembly of actin for microspike formation. EMBO J. 1998; 17(22):6516-26. [PMID: 9822597]
- Higgs HN, Blanchoin L, and Pollard TD. Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization. Biochemistry 1999; 38(46):15212-22. [PMID: 10563804]
- Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, and Carlier MF. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 1999; 146(6):1319-32. [PMID: 10491394]
- Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, and Pollard TD. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A. 1999; 96(7):3739-44. [PMID: 10097107]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, and Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 1999; 97(2):221-31. [PMID: 10219243]
- Yarar D, To W, Abo A, and Welch MD. The Wiskott-Aldrich syndrome protein directs actin-based motility by stimulating actin nucleation with the Arp2/3 complex. Curr. Biol. 1999; 9(10):555-8. [PMID: 10339430]
- Miki H, Yamaguchi H, Suetsugu S, and Takenawa T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature 2000; 408(6813):732-5. [PMID: 11130076]
- Shi Y, Dong B, Miliotis H, Liu J, Alberts AS, Zhang J, and Siminovitch KA. Src kinase Hck association with the WASp and mDia1 cytoskeletal regulators promotes chemoattractant-induced Hck membrane targeting and activation in neutrophils. Biochem. Cell Biol. 2009; 87(1):207-16. [PMID: 19234535]
- Shi Y, Zhang J, Mullin M, Dong B, Alberts AS, and Siminovitch KA. The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J. Immunol. 2009; 182(6):3837-45. [PMID: 19265163]
- Sarmiento C, Wang W, Dovas A, Yamaguchi H, Sidani M, El-Sibai M, Desmarais V, Holman HA, Kitchen S, Backer JM, Alberts A, and Condeelis J. WASP family members and formin proteins coordinate regulation of cell protrusions in carcinoma cells. J. Cell Biol. 2008; 180(6):1245-60. [PMID: 18362183]
- Le Clainche C, and Carlier M. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 2008; 88(2):489-513. [PMID: 18391171]
- Campellone KG, and Welch MD. A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 2010; 11(4):237-51. [PMID: 20237478]
- Machesky LM, and Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8(25):1347-56. [PMID: 9889097]
- Sirotkin V, Beltzner CC, Marchand J, and Pollard TD. Interactions of WASp, myosin-I, and verprolin with Arp2/3 complex during actin patch assembly in fission yeast. J. Cell Biol. 2005; 170(4):637-48. [PMID: 16087707]
- Ramesh N, Antón IM, Hartwig JH, and Geha RS. WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl. Acad. Sci. U.S.A. 1997; 94(26):14671-6. [PMID: 9405671]
- Kinley AW, Weed SA, Weaver AM, Karginov AV, Bissonette E, Cooper JA, and Parsons JT. Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr. Biol. 2003; 13(5):384-93. [PMID: 12620186]
- Lee S, Lee K, Hwang S, Kim SH, Song WK, Park ZY, and Chang S. SPIN90/WISH interacts with PSD-95 and regulates dendritic spinogenesis via an N-WASP-independent mechanism. EMBO J. 2006; 25(20):4983-95. [PMID: 16990791]
- Rajmohan R, Meng L, Yu S, and Thanabalu T. WASP suppresses the growth defect of Saccharomyces cerevisiae las17Delta strain in the presence of WIP. Biochem. Biophys. Res. Commun. 2006; 342(2):529-36. [PMID: 16488394]
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, and Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 2002; 418(6899):790-3. [PMID: 12181570]
- Suetsugu S, Kurisu S, Oikawa T, Yamazaki D, Oda A, and Takenawa T. Optimization of WAVE2 complex-induced actin polymerization by membrane-bound IRSp53, PIP(3), and Rac. J. Cell Biol. 2006; 173(4):571-85. [PMID: 16702231]
- Mattila PK, Pykäläinen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, and Lappalainen P. Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J. Cell Biol. 2007; 176(7):953-64. [PMID: 17371834]
- Bear JE, and Gertler FB. Ena/VASP: towards resolving a pointed controversy at the barbed end. J. Cell. Sci. 2009; 122(Pt 12):1947-53. [PMID: 19494122]
- Gertler FB, Niebuhr K, Reinhard M, Wehland J, and Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell 1996; 87(2):227-39. [PMID: 8861907]
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, and Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 2002; 109(4):509-21. [PMID: 12086607]
- Breitsprecher D, Kiesewetter AK, Linkner J, Urbanke C, Resch GP, Small JV, and Faix J. Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation. EMBO J. 2008; 27(22):2943-54. [PMID: 18923426]
- Lebrand C, Dent EW, Strasser GA, Lanier LM, Krause M, Svitkina TM, Borisy GG, and Gertler FB. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 2004; 42(1):37-49. [PMID: 15066263]
- Chesarone MA, and Goode BL. Actin nucleation and elongation factors: mechanisms and interplay. Curr. Opin. Cell Biol. 2009; 21(1):28-37. [PMID: 19168341]
- Breitsprecher D, Kiesewetter AK, Linkner J, Vinzenz M, Stradal TEB, Small JV, Curth U, Dickinson RB, and Faix J. Molecular mechanism of Ena/VASP-mediated actin-filament elongation. EMBO J. 2011; 30(3):456-67. [PMID: 21217643]
- Fradelizi J, Noireaux V, Plastino J, Menichi B, Louvard D, Sykes C, Golsteyn RM, and Friederich E. ActA and human zyxin harbour Arp2/3-independent actin-polymerization activity. Nat. Cell Biol. 2001; 3(8):699-707. [PMID: 11483954]


