Andrea RAVASIO
Alumni, Mechanobiology Institute, National University of Singapore
mbiar@nus.edu.sg, and.ravasio@gmail.com
Level 10 T-Lab
National University of Singapore
5A Engineering Drive 1
Singapore 117411
Public Profiles
LinkedIn, ResearchGate
Illuminating the Contacts
Using superresolution imagining to map adherens junction machinery
The Mending Tissue
Cellular instructions for tissue repair
Andrea Ravasio
MBI Alumni, May 2017
Principal Investigator
Research Interests
Technology Innovation for Mechanobiology
My research is focused on the mechanical properties of migratory cells, and the role of physical forces in the regulation of biological systems; especially in physiological, and disease states. To approach these topics from novel perspectives, I enjoy devoting much time and effort to the development of new methods.
Research Philosophy
“Truth in science can be defined as the working hypothesis best suited to open the way to the next better one.” —Konrad Lorentz
Research Areas
Cell migration, tissue dynamics, cell biophysics and biomechanics, bioengineering.
Projects
Cell migration and biomechanics of cancer cells
Cell migration is a vital process during morphogenesis, tissue repair and wound healing. It is also fundamental to cancer progression, with the activation of cell motility in transformed cells leading to the establishment of population heterogeneity, and drug resistance. In collaboration with the Genome Institute of Singapore (A-Star), and the Cancer Science Institute of Singapore (NUS/NUH), we are characterizing the migration properties of cancer cells with different metastatic potential. Furthermore, we are analysing the dynamics of receptor kinases at the cell cortex in response to ligand binding. Combinatorial analysis of these parameters allows us to determine the invasion potential of cancer cells at a single cell level and to select potentially invasive cells from heterogeneous populations.
Collaborators: Virgile Viasnoff (MBI/CNRS), Jay Goves (MBI/Berkley), Ramanuj DasGupta (GIS, A-Star), Huang Yun Ju Ruby (CSI, NUS/NUH).
Read more: Publication in preparation
Tissue dynamics
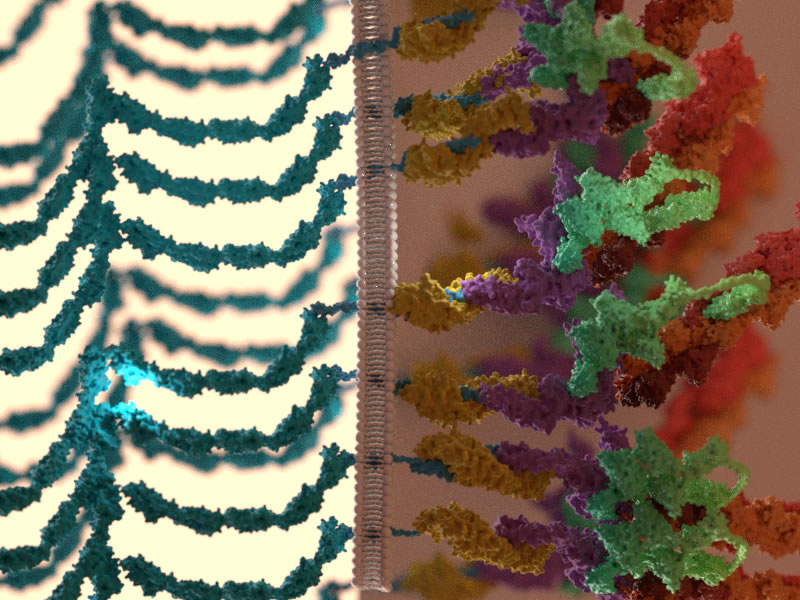
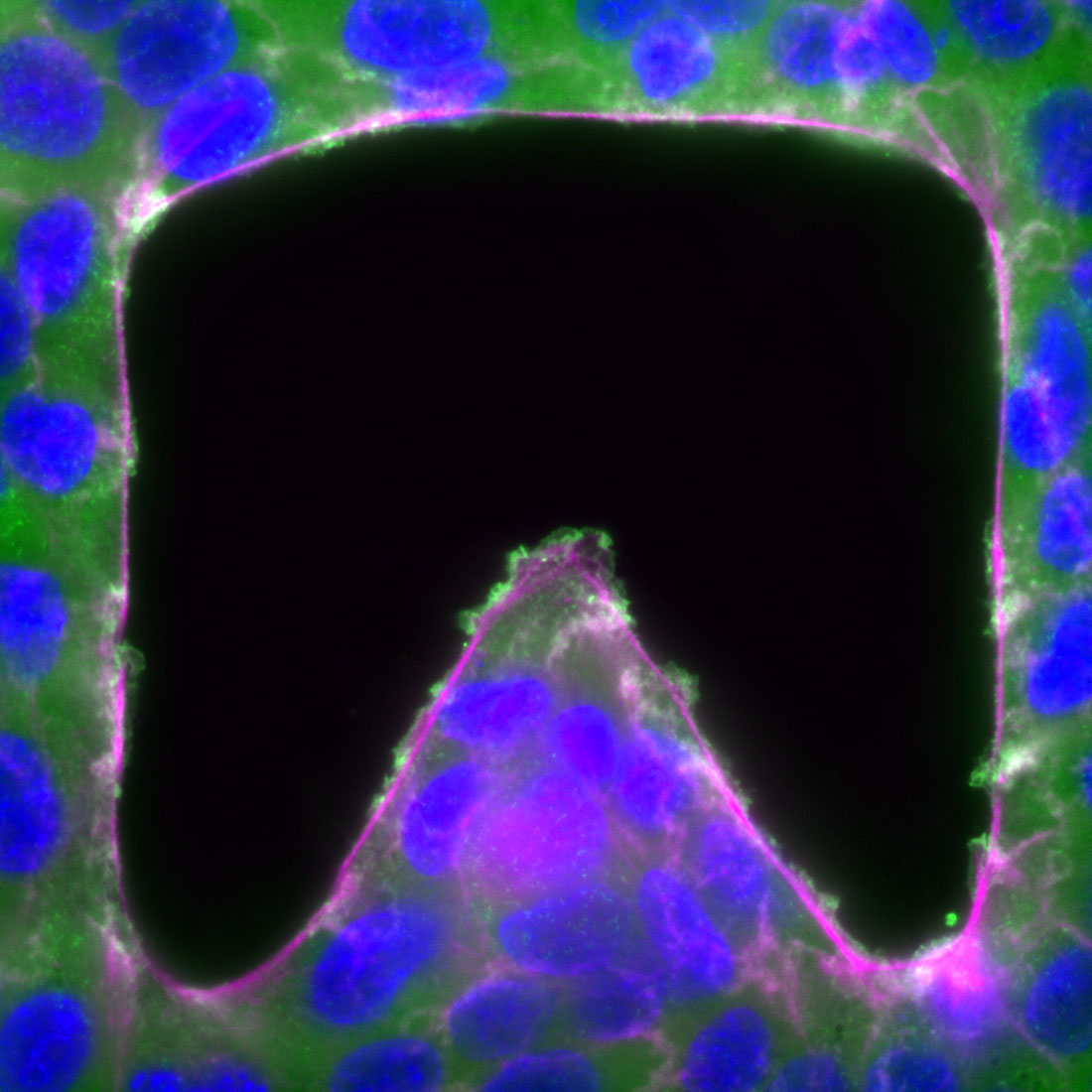
 During morphogenesis and tissue repair, epithelial cells interact with neighbouring cells, and with the substrate, in order to maintain or restore proper tissue function. In this project, we investigate the effect of tissue geometry, cell-cell interactions, and cell-substrate interactions, on the migration properties of epithelial cells. In collaboration with physicists and mathematicians, we have developed computational models that describe experimental observations in quantitative terms. Using this framework, we are currently studying the molecular organization of cell-cell adhesions by super resolution and FRET microscopy.
During morphogenesis and tissue repair, epithelial cells interact with neighbouring cells, and with the substrate, in order to maintain or restore proper tissue function. In this project, we investigate the effect of tissue geometry, cell-cell interactions, and cell-substrate interactions, on the migration properties of epithelial cells. In collaboration with physicists and mathematicians, we have developed computational models that describe experimental observations in quantitative terms. Using this framework, we are currently studying the molecular organization of cell-cell adhesions by super resolution and FRET microscopy.
Collaborators: Benoit Ladoux (MBI/Institut Jacques Monod), Luis Almeida (Sorbonne Universites), Nir Gov (Weizmann Institute), Cristina Bertocchi (MBI), Pakorn Yony Kanchanawong (MBI), Chwee Teck Lim (MBI).
Read more: Ravasio et al., Nature Communications, 2015; Ravasio et al., Integrative Biology, 2015; Tarle et al., Integrative Biology, 2015; Vedula et al., Physiology, 2013.
Bioengineering
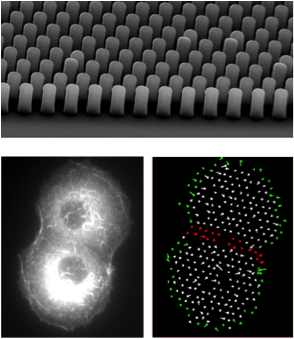 Understanding biophysical phenomena often requires approaching our research from novel perspectives. To this end, we continue to design and implement new methodologies, devices and approaches. In recent years, we have introduced micro-pillars made of an index match polymer (MyPoly 134) to measure traction forces, and simultaneously visualize cellular structures at high resolution. We have also introduced a new device (IR-Live) that can obtain chemical maps of living cells by FTIR, at high spatial resolution. Currently, we are using microfabrication techniques to test the effect of substrate geometry, rheology and texture on tissue organization. Finally, we are developing a new device that allows us to measure traction force whilst simultaneously super-resolving cellular structures.
Understanding biophysical phenomena often requires approaching our research from novel perspectives. To this end, we continue to design and implement new methodologies, devices and approaches. In recent years, we have introduced micro-pillars made of an index match polymer (MyPoly 134) to measure traction forces, and simultaneously visualize cellular structures at high resolution. We have also introduced a new device (IR-Live) that can obtain chemical maps of living cells by FTIR, at high spatial resolution. Currently, we are using microfabrication techniques to test the effect of substrate geometry, rheology and texture on tissue organization. Finally, we are developing a new device that allows us to measure traction force whilst simultaneously super-resolving cellular structures.
Collaborators: Gianluca Grenci (MBI), Virgile Viasnoff (MBI/CNRS), Benoit Ladoux (MBI/Institut Jacques Monod), Cristina Bertocchi (MBI).
Read more: Ravasio et al., Acta Biomaterialia, 2015; Vedula et al., Methods in Cell Biology, 2014; Braida et al., Lab on a chip, 2016.
Biography
Throughout my research career, I have applied highly interdisciplinary approaches aimed at elucidating the interplay between forces and living matter. After my undergraduate studies in biology and physiology at the University of Milan (Italy), I conducted an experiment-based master thesis in Membrane Molecular Physiology (Medical University of Innsbruck, Austria), where I investigated protein-protein and protein-membrane interactions in the context of cell volume regulation using the FRET technique. Thereafter, I pursued a PhD at the Department of Physiology and Biomedical Physics (Medical University of Innsbruck, Austria). During my PhD, I developed and employed a number of pioneering methods to study the physiology of the lung. With my colleagues, we demonstrated for the first time the physiologically important interplay between epithelial cells and the mechanical forces at the alveolar air-liquid interface.
Additionally, I have studied the biophysical properties of lung surfactant during adsorption and interfacial reorganization. Afterwards, I moved to the School of Biological Science at the Nanyang Technological University (NTU, Singapore). During this time at NTU, I studied the molecular events leading up to red blood cell invasion by malaria parasites. Currently, I’m enjoying the stimulating environment of the Mechanobiology Institute (Singapore) where I’m working as senior research fellow in the lab of Virgile Viasnoff, and I closely collaborate with Benoit Ladoux, Jay Groves, Cristina Bertocchi and Gianluca Grenci.
Education
PhD Department of Physiology and Biomedical Physics, Medical University of Innsbruck, Austria
Recent Publications
- Morales-Camilo N, Liu J, Ramírez MJ, Canales-Salgado P, Alegría JJ, Liu X, Ong HT, Barrera NP, Fierro A, Toyama Y, Goult BT, Wang Y, Meng Y, Nishimura R, Fong-Ngern K, Low CSL, Kanchanawong P, Yan J, Ravasio A, and Bertocchi C. Alternative molecular mechanisms for force transmission at adherens junctions via β-catenin-vinculin interaction. Nat Commun 2024; 15(1):5608. [PMID: 38969637]
- Hirata H, Nakazawa N, Hirashima T, and Ravasio A. Editorial: Multicellularity: Views from cellular signaling and mechanics. Front Cell Dev Biol 2023; 11:1172921. [PMID: 36994099]
- Joy-Immediato M, Ramirez MJ, Cerda M, Toyama Y, Ravasio A, Kanchanawong P, and Bertocchi C. Junctional ER Organization Affects Mechanotransduction at Cadherin-Mediated Adhesions. Front Cell Dev Biol 2021; 9:669086. [PMID: 34222239]
- Arora A, Niño JLG, Myaing MZ, Chia S, Arasi B, Ravasio A, Huang RY, Dasgupta R, Biro M, and Viasnoff V. Two high-yield complementary methods to sort cell populations by their 2D or 3D migration speed. Mol Biol Cell 2020;:mbcE20070466. [PMID: 33085550]
- Tan X, Ravasio A, Ong HT, Wu J, and Hew CL. White spot syndrome viral protein VP9 alters the cellular higher-order chromatin structure. FASEB Bioadv 2020; 2(4):264-279. [PMID: 32259052]
- Grenci G, Bertocchi C, and Ravasio A. Integrating Microfabrication into Biological Investigations: the Benefits of Interdisciplinarity. Micromachines (Basel) 2019; 10(4). [PMID: 30995747]
- Chen T, Callan-Jones A, Fedorov E, Ravasio A, Brugués A, Ong HT, Toyama Y, Low BC, Trepat X, Shemesh T, Voituriez R, and Ladoux B. Large-scale curvature sensing by directional actin flow drives cellular migration mode switching. Nat Phys 2019; 15:393-402. [PMID: 30984281]
- Bertocchi C, Wang Y, Ravasio A, Hara Y, Wu Y, Sailov T, Baird MA, Davidson MW, Zaidel-Bar R, Toyama Y, Ladoux B, Mege R, and Kanchanawong P. Nanoscale architecture of cadherin-based cell adhesions. Nat. Cell Biol. 2016;. [PMID: 27992406]
- Ruprecht V, Monzo P, Ravasio A, Yue Z, Makhija E, Strale PO, Gauthier N, Shivashankar GV, Studer V, Albiges-Rizo C, and Viasnoff V. How cells respond to environmental cues - insights from bio-functionalized substrates. J. Cell. Sci. 2016;. [PMID: 27856508]
- Birarda G, Ravasio A, Suryana M, Maniam S, Holman HN, and Grenci G. IR-Live: fabrication of a low-cost plastic microfluidic device for infrared spectromicroscopy of living cells. Lab Chip 2016; 16(9):1644-51. [PMID: 27040369]