How Structural Imbalance Drives Inflammatory Signaling in Senescent Cells
HIF-1α-activation in SASP is a defining feature of the SASP induced by diverse stressors, acting independently of micronuclei generation and cGAS/STING activation.
A study from the Low BC Lab at MBI demonstrate how targeting HIF-1α-driven pathways may open new avenues for targeting aging tissues and cancer without broadly suppressing immune responses.
As cells age or experience severe stress, many enter a state called cellular senescence. Senescent cells permanently stop dividing, but they remain biologically active, releasing a mixture of inflammatory molecules, growth factors, and enzymes known as the Senescence-Associated Secretory Phenotype (SASP). This secretory behavior influences tissue remodeling, aging, and cancer progression. While SASP is often discussed in biochemical terms, a new study published in Molecular Biology of the Cell, led by Celestine Ho and Selwin Wu from the Mechanobiology Institute, NUS and Lin Deng, Institute of Molecular Physiology, Shenzhen Bay Laboratory, reveals that physical and structural stress inside cells plays a central role in shaping this response.
Cells can become senescent after repeated cell division, DNA damage, oncogene activation, or defects during cell division. By comparing gene expression across these different forms of senescence, a commonality was discovered. Genes typically activated when cells experience low oxygen levels were constantly switched on in senescent cells – even when oxygen was not limited – and in the center of this response is HIF-1α (hypoxia-inducible factor 1 alpha), a transcription factor best known for regulating how cells respond to low oxygen.
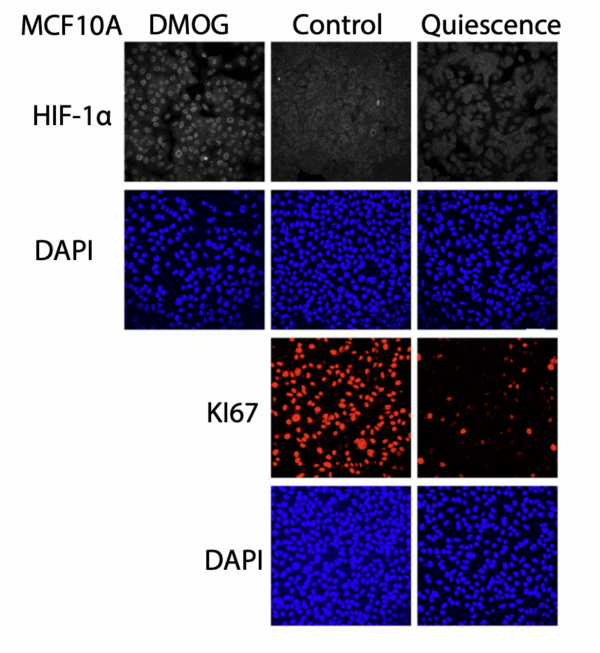
Figure showing that HIF-1α is not induced in quiescent cells. Source: https://doi.org/10.1091/mbc.E24-10-0445
Importantly, HIF-1α was activated in senescent cells even under normal oxygen conditions, and it was absent in quiescent cells – which are temporarily resting but able to divide again. This distinction identifies HIF-1α as a marker of permanent, stress-induced aging rather than reversible cell cycle arrest.
The authors also focused on a less conventional trigger of senescence, centrosome amplification, providing a key insight on how mechanical disruption inside the cell can drive this response. Centrosomes organize the microtubule cytoskeleton, which governs cell shape, force distribution, and division mechanics. When centrosome numbers amplify, the cytoskeleton becomes physically imbalanced. This structural disruption generates mechanical and metabolic stress, including elevated reactive oxygen species. In these cells, the SASP was not driven primarily by the classical inflammatory regulator NF-κB. Instead, the inflammatory and secretory response was dominated by HIF-1α – effectively converting a mechanical disturbance into a gene expression program associated with inflammation.
The video shows that conditioned media from epithelial cells with centrosome amplification stimulates the leading edge movement of control cells. Live imaging of control epithelial cells without centrosome amplification before (left) or after (right) centrosome amplification conditioned media swap. Source: https://doi-org.libproxy1.nus.edu.sg/10.1091/mbc.E24-10-0445
The factors released by these senescent cells were shown to influence neighboring cells, increasing cell movement and invasive behavior in laboratory models, processes that are relevant to cancer progression.
The study also challenges another widely held idea: that micronuclei, small DNA-containing structures formed during faulty cell division, trigger inflammatory signaling through the cGAS/STING immune pathway. By analyzing individual cells, the researchers show that micronuclei generated by centrosome amplification rarely activate this pathway. Instead, inflammation can arise independently of DNA sensing and be driven by structural stress and HIF-1α signaling.
Together, these findings repositions senescence under the mechanobiological lens, where SASP appears to be fundamentally linked to physical disruption of cellular architecture and HIF-1α activation across diverse senescence triggers. Understanding how cells convert mechanical stress into SASP and targeting HIF-1α-driven pathways may open new avenues for targeting aging tissues and cancer without broadly suppressing immune responses.