Chii Jou (Joe) CHAN
Assistant Professor, Mechanobiology Institute, National University of Singapore
dbschii@nus.edu.sg
10-03F
Level 10 T-Lab
National University of Singapore
5A Engineering Drive 1
Singapore 117411
Laboratory website
Mammalian Development and Tissue Hydraulics Laboratory
Affiliations
Department of Biological Sciences, National University of Singapore
She is a Scientist: Confidence Formed by Curiosity Over Time
February 11th celebrates The International Day of Women and Girls in Science to promote full, equal access and participation in STEM fields. MBI talks to two of our posdoctoral research fellows about their career as a woman in science.
MBI welcomes new Principal Investigator, Chii Jou Chan
MBI would like to welcome Chii Jou (Joe) Chan as a new Principal Investigator starting in January 2021, with a joint appointment as Assistant Professor, Department of Biological Science, NUS.
Chii Jou (Joe) Chan
Principal Investigator
Research Areas
Mammalian oogenesis, embryogenesis, tissue hydraulics, mechanochemical feedback in tissue self-organisation
Research Interests
Our research aims to address the following questions in developmental biology: How are tissue size and shape precisely controlled during early mammalian development? Conversely how is tissue geometry sensed and transmitted to the cellular level to impact cellular/molecular functions? How are mechanics and biochemical signalling integrated across multiple scales to ensure robust morphogenesis and patterning?
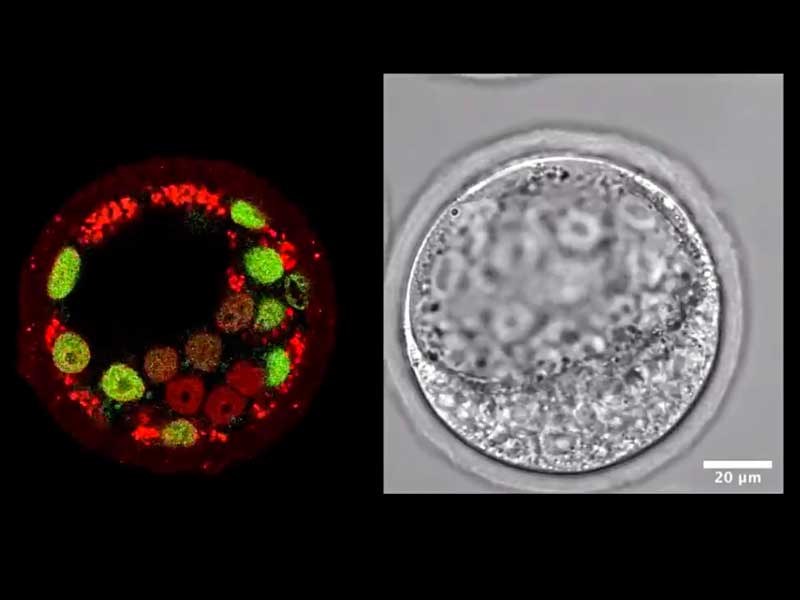
Research from the Chan Lab.
To address these fundamental questions, we focus on understanding mammalian oogenesis, the formation of eggs (oocytes) that provide the bulk genetic and cytoplasmic materials for successful reproduction. The growth of functional oocytes is characterised by their correct size and number, which are tightly controlled during oogenesis. However the underlying mechanisms, particularly how the mechanics of the oocyte microenvironment can influence its development, remain poorly understood. In our lab, we will develop biomechanical tools to map out the mechanical interactions between the oocyte, its surrounding somatic cells and the ovarian tissue. We will also utilise advanced deep tissue imaging techniques to study follicle dynamics in vivo. Eventually we will combine these insights with synthetic biomimetics, biophysical and genetic manipulations, and mathematical modelling to understand the mechanical and molecular mechanisms regulating oogenesis.
An important, albeit understudied aspect is the role of tissue hydraulics in development. We have recently shown that luminal pressure plays a critical role in regulating the tissue size and cell fate specification of mouse blastocysts (Nature 2019, https://doi.org/10.1038/s41586-019-1309-x). In mammalian oogenesis, a similar process occurs where a fluid-filled cavity emerges at the antral follicle stage. Our lab will study the dynamics and mechanisms of luminogenesis, and investigate how luminal pressure and signalling collectively influence the oocyte development. Another aspect where hydraulics may play a role in oogenesis is the formation of germline cysts in the fetal ovaries. How these interconnected germ cells undergo death and cyst breakup prior to birth remains unknown. We hypothesize that this process may be driven by pressure-induced fluid exchange between the germ cells, and will test this hypothesis using quantitative imaging, biophysics and developmental genetics.
The ultimate goal of the lab is to extend these understanding to other mammalian species and identify common principles underlying oogenesis. A quantitative understanding of the mechanical aspects of oogenesis will deepen our understanding of reproductive biology and ageing and have important implications for regenerative medicine and tissue engineering.
Teaching
LSM2234 – Introduction to Quantitative Biology
LSM3236 – Pattern Formation and Self-organisation in Biology
LSM4252 – Reproductive Biology
Consultation hours: Friday 2-4pm
Education
B.A., M.Sc. (First Class Honours), University of Cambridge, UK
M.Phil., University of Cambridge, UK
Ph.D., University of Cambridge, UK
Biography
Chii Jou (Joe) Chan was trained in theoretical soft matter physics at the University of Cambridge (B.A., M.Phil.). For his Ph.D. with Prof. Jochen Guck at Cambridge and TU Dresden (Germany), he studied the mechanical and optical properties of living cells and nuclei, using biomechanical tools (optical stretcher, microfluidics) and biophotonics. Inspired by how physical forces shape early development of living organisms, he joined the group of Dr. Takashi Hiiragi as an EIPOD fellow at EMBL Heidelberg (Germany), where he made a major discovery in hydraulic regulation of mouse embryo size and cell fate specification. His interdisciplinary productivity is reflected in the diversity of his collaborators (cell and developmental biologists, experimental biophysicists, theorists) across the world. Joe was awarded the Singaporean Teaching and Academic Research Talent (START) Inauguration Grant from the MOE and NUS, and will launch his research group at MBI and NUS Department of Biological Sciences in Jan 2021.
Recent Publications
- Biswas A, Lou YT, Ng BH, … & Chan CJ. Surface mechanics and compressive stress impact mammalian follicle development. Nat. Communications. (2025)
- Chan CJ. Editorial for special issue: Environmental control of oogenesis and ovulatory dynamics. Seminars in Cell & Developmental Biology. (2025)
- Jaeschke A, Hepburn MS, Mowla A, Kennedy BF, Chan CJ. Three-dimensional quantitative micro-elastography reveals alterations in spatial elasticity patterns in murine ovaries during ageing. Communications Biology. (2025)
- Leong KW, Lou Y, … & Chan CJ. Critical phenomenon underlies de novo luminogenesis during mammalian follicle development. bioRxiv. (2025)
- Tomida K., Ong HT., Young JL., Chan CJ. Capturing ovarian dynamics through spatial profiling of the mechano-microenvironment. Seminars in Cell & Developmental Biology. (2025)
- Wohland T., Saunders T.E., Chan CJ. Developmental biophysics. Biophysical Journal. (2025)
- Turley J, Leong KW, Chan CJ. Novel imaging and biophysical approaches to study tissue hydraulics in mammalian folliculogenesis. Biophysical Reviews. (2024)
- Jaeschke A, Hepburn MS, Mowla A, Kennedy BF, Chan CJ. Three-dimensional quantitative micro-elastography reveals alterations in spatial elasticity patterns of follicles and corpora lutea in murine ovaries during ageing. bioRxiv. (2024)
- Biswas A, Lou YT, Ng BH, Tomida K, Darpe S, Wu Z, Lu TB, Bonne I, Chan CJ. Theca cell mechanics and tissue pressure regulate mammalian ovarian folliculogenesis. bioRxiv. (2024)
- Hepburn M, Jaeschke A, Mowla A, Chan CJ, Kennedy BF. Three-dimensional characterization of murine ovary elasticity using quantitative micro-elastography. Optical Elastography and Tissue Biomechanics XI. (2024), PC128440B
- Bevilacqua C, Gomez JM, Fiuza U-M, Chan CJ, Wang L, Hambura S, Eguren M, Ellenberg J, Diz-Muñoz A, Leptin M, Prevedel R. High-resolution line-scan Brillouin microscopy for live-imaging of mechanical properties during embryo development. Nature Methods. (2023)
- Biswas A, Ng BH, Prabhakaran V, Chan CJ. Squeezing the eggs to grow: The mechanobiology of mammalian folliculogenesis. Front. Cell Dev. Biol. (2022)
- Chan CJ, Hirashima T. Tissue Hydraulics in Reproduction. Seminars in Cell & Developmental Biology (2022)
- Chan CJ, Bevilacqua C, Prevedel R. Mechanical mapping of mammalian follicle development using Brillouin microscopy. Comm. Biology (2021) 4 (1133)
- Yang Q, Xue S-L, Chan CJ, Rempfler M, Vischi D, Gutierrez F M, Hiiragi T, Hannezo E, Liberali. Cell fate coordinates mechano-osmotic forces in intestinal crypt morphogenesis. Nature Cell Biology (2021).
- Roffay C*, Chan CJ*, Guirao B, Hiiragi T, Graner F. Inferring cell junction tension and pressure from cell geometry. Development (2021) 148 (18) dev192773.
- Bevilacqua C, Hambura S, Wang L, Chan CJ, Eguren M, Gomez Elliff JM, Diz-Muñoz A, Prevedel R. High-resolution line-scanning Brillouin microscopy for fast and low phototoxicity live-imaging of mechanical properties in biology. Elastography and Tissue Biomechanics VIII (2021), 11645
- Chan CJ, Hiiragi T. Integration of luminal pressure and signalling in tissue self-organisation. Development (2020) 147 (5), 1-10
- Ryan AQ, Chan CJ, Graner F, Hiiragi T. Lumen expansion facilitates epiblast-primitive endoderm fate specification during mouse blastocyst formation. Developmental Cell (2019) 51, 1-14.
- Chan CJ, Costanzo M, Ruiz-Herrero T, Mönke G, Petrie R, Bergert M, Diz-Munoz A, Mahadevan L, Hiiragi T. Hydraulic control of mammalian embryo size and cell fate. Nature (2019) 571:112-116
- Chan CJ, Heisenberg C-P, Hiiragi T. Coordination of morphogenesis and cell fate specification in development. Current Biology. (2017) 27(18):R1024-R1035.
- Chan CJ, Hiiragi T. Keeping in touch to differentiate. Developmental Cell (2017) 43(2):113-114
- Chan CJ, Li W, Cojoc G, Guck J, Volume transitions of isolated cell nuclei induced by rapid temperature increase. Biophysical Journal (2017) 112(6):1063-1076
- Schürmann M, Scholze J, Müller P, Guck J, Chan CJ. Cell nuclei have lower refractive index and mass density than cytoplasm. Journal of Biophotonics (2016) 9(10): 1068-1076.
- Chan CJ, Ekpenyong AE, Golfier S, Li W, Chalut KJ, Otto O, Elgeti J, Guck J, Lautenschläger F. Myosin II activity softens cells in suspension. Biophysical Journal (2015) 108(8): 1856–1869
- Schürmann M, Scholze J, Müller P, Chan CJ, Ekpenyong AE, Chalut KJ, Guck J. Refractive index measurements of single, spherical cells using digital holographic microscopy. Methods in Cell Biology (2015) 125:143-159.
- Chan CJ, Whyte G, Boyde L, Salbreux G, Guck J. Impact of heating on passive and active biomechanics of suspended cells. Interface Focus (2014) 4, 20130069.
- Chalut KJ, Höpfler M, Lautenschläger F, Boyde L, Chan CJ, Ekpenyong AE, Martinez-Arias A, Guck J. Chromatin decondensation and nuclear softening accompany Nanog downregulation in embryonic stem cells. Biophysical Journal (2012) 103(10): 2060-2070.
- Chan CJ, Terentjev EM. Non-equilibrium statistical mechanics of liquid crystals: relaxation, viscosity and elasticity. Journal of Physics A (2007) 40 R103-R148 Topic Review.
- Chan CJ, Terentjev EM. Non-equilibrium statistical mechanics of nematic liquids. IMA Volumes in Mathematics and Its Applications, Modeling of Soft Matter (2005) 141:27-84.
Lab Members
Yiwen Tang
Research Fellow, Chan Group
Neha Paddillaya
Research Fellow, Chan Group
Tan Jue Yu Kelly
PhD Student, Class of August 2024, Chan Group
Cao Gefei
PhD Student, Class of August 2024, Hirashima Group, Chan Group
Jake Matthew Turley
Schmidt AI In Science Postdoctoral Fellow, Chan Group
Kosei Tomida
PhD Student, Class of August 2023, Chan Group
Apoorva Shivankar
Research Assistant, Chan Group
Arikta Biswas
Research Fellow, Chan Group
Ng Boon Heng
PhD Student, Class of August 2021, Chan Group
Leong Kim Whye
Research Fellow, Class of August 2019, Chan Group
Lab Alumni
Xixun Lu
SPS Intern, Chan Group
Lee Chin Hao
FYP Student, Chan Group
Sukhada Darpe
Intern, Chan Group
Hugo Raveton
Intern, Chan Group
Nicole Loh Kit Ying
Alumni, Chan Group
Anna Jaeschke
Research Fellow, Chan Group
Vinod S/O Prabhakaran
Research Assistant, Young Group, Chan Group
Zihao Wu
Alumni, Chan Group
Wan Jun Gan
Research Fellow, Chan Group
Lou Yuting
Research Fellow, Chan Group