CHII JOU (JOE) CHAN LABORATORY
Mammalian Development and Tissue Hydraulics Laboratory
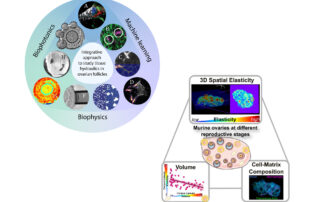
Our research aims to address two broad questions in developmental biology: How are tissue size and shape precisely controlled during early mammalian development? How is tissue geometry sensed and transmitted to the cellular level to impact cellular functions? How are mechanics and biochemical signalling integrated across multiple scales to ensure robust morphogenesis and patterning?
To address these fundamental questions, we focus on understanding mammalian follicle development, which is critical for the maturation of functional eggs for successful reproduction. We aim to identify the mechanobiology principles underlying mammalian follicle growth, and decipher the roles of tissue mechanics and fluid forces in oocyte quality control, using ex vivo mouse ovaries and follicle culture. A quantitative and mechanical understanding of folliculogenesis will deepen our understanding of reproductive biology and ageing, with important implications for assisted reproductive technology (ART), infertility treatment and mechano-therapeutics.
Lab Meeting with the Chan Lab – the Node
The Chan Lab sits down with the Node for to chat about lab life!
The Chan lab celebrates two new papers!
Congratulations to the teams!
Congratulations to Kim Whye Leong for presenting excellent work at the Imaging Mouse Development Workshop held at EMBL Heidelberg!
Congratulations to Kim Whye Leong for presenting excellent work at the Imaging Mouse Development Workshop held at EMBL Heidelberg from 2 to 5 Sept 2024. Thanks for all the feedback from the expert audience!
CHII JOU (JOE) CHAN LABORATORY
Research
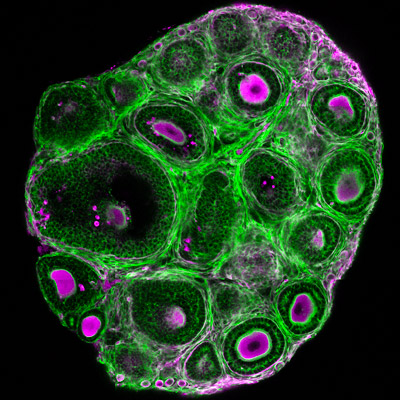
Squeezing the eggs: Mechanical control of follicle development
While the follicular stages have been well characterized by histological criteria, the mechanisms underlying follicular growth remain poorly understood, partly due to a lack of understanding of the mechanical interactions between the oocyte and its surrounding somatic cells and extracellular matrix (ECM). We will develop novel biophysical tools to measure and study these mechanical processes. In combination with deep tissue imaging and physical and genetic manipulations, we aim to unravel key mechano-signaling pathways controlling follicle morphogenesis and the oocyte quality, as proposed in our recent review (Front. Cell Dev. Biol. 2022). One approach is Brillouin microscopy, which we have shown to reveal mechanical compartmentalization during follicle and embryo maturation (Comm. Biol. 2021, Nat. Methods 2023).
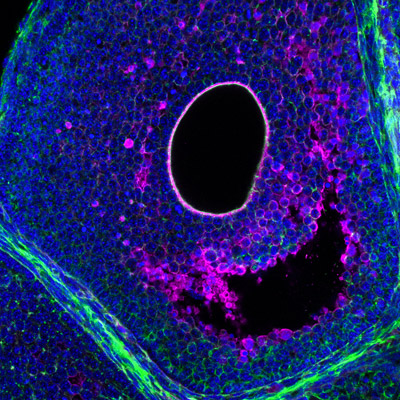
Tissue hydraulics in mammalian development
An important, albeit understudied aspect is the role of tissue hydraulics in development and reproduction (see our review in Semin. Cell Dev. Biol. 2022). We have recently shown that luminal pressure and signalling play a critical role in regulating the tissue size and cell fate specification of mouse blastocysts (Nature 2019, Dev. Cell 2019), and proposed a unifying framework to understand the interplay between luminal mechanics, signaling and cell fate during tissue morphogenesis (Development 2020). We have also worked with others to reveal the mechano-osmotic control of intestinal organoid morphogenesis (Nat. Cell Biology 2021). Interestingly, during mammalian folliculogenesis, a similar process occurs where a fluid-filled cavity emerges at the antral follicle stage. We will study the dynamics and mechanisms of luminogenesis, and investigate how luminal pressure and signalling collectively influence the oocyte development, using deep tissue imaging, machine learning, biophysical modelling and genetics.
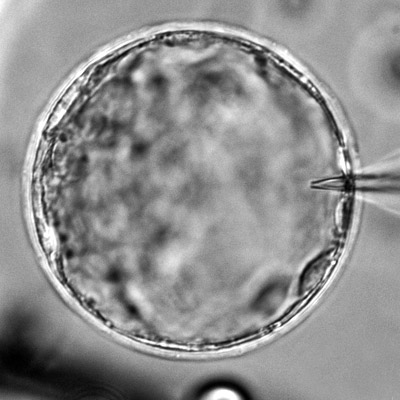
Alterations in tissue mechanics and hydraulics during ovarian ageing
Current ART such as in vitro fertilisation (IVF) and in vitro maturation (IVM) are costly and achieve a low success rate of 20-37%. One reason could be the removal of the follicle microenvironment that provides crucial mechanical signals that ensure oocyte health. Specifically, changes in the ovarian ECM mechanics during ovarian ageing could impact oocyte quality through dysregulated mechano-signalling pathways. We will quantify these mechanical changes at both the intra- and extra-follicular environment during ovarian ageing, and correlate these findings with the oocyte function and ovarian reserve metrics. Infertility could also be associated with impaired antrum formation that leads to poor ovulation, which we will investigate by tracking luminogenesis, luminal pressure and follicle wall stress during reproductive ageing. These projects will entail the use of biophotonics/ mechanical tools such as quantitative micro-elasticity, in vivo force sensors and stress inference that we have applied in the past (Nat. Methods 2023, Development 2021, Nature 2019).
CHII JOU (JOE) CHAN LABORATORY
Current lab members

CHII JOU (JOE) CHAN LABORATORY
Current lab members

Ng Boon Heng
Boon Heng joins the Chan Lab as a PhD student, having obtained his B.Sc. in Life Sciences from the National University of Singapore in 2021. His previous research spans from C. elegans embryogenesis to zebrafish somite formation. Using a mouse model, he wishes to explore how mechanics and biochemical signalling are tightly coordinated to drive robust tissue patterning in pre-antral follicle development. Learn more

Arikta Biswas
During her PhD at IISER Kolkata, Arikta developed novel imaging techniques to quantify membrane tension with high spatio-temporal resolution, and relate these findings to cell mechanosensing and migration. At MBI, she will apply new biophysical approaches and quantitative imaging to understand how tissue compaction and basement membrane mechanics guide cell proliferation during follicle morphogenesis. Learn more

Apoorva Shivankar
Apoorva Shivankar joins the Chan lab as a Research Assistant, having obtained her Master’s degree in Clinical Biochemistry in India. She previously worked at SingHealth, focusing on how surface topographies impact cell migration and differentiation during tissue regeneration. In the Chan lab, she will investigate how changes in macrophage-ECM interactions contribute to ovarian ageing. Learn more

Leong Kim Whye
Kim Whye will be graduating with a PhD from the Mechanobiology Institute (MBI), National University of Singapore, Singapore in 2024. During his PhD, he focused on using an in vitro bile duct model to study morphogenetic events during development and apply them in a disease context. In the Chan lab, he will be investigating antrum formation, probing its mechanochemical functions during follicle development and oocyte maturation using advanced 3D microscopy and tools of force measurement and manipulation. Learn more

Kosei Tomida
Kosei joins the Chan Lab as a PhD student, having obtained his M.Eng. from Japan where he studied chondrogenic differentiation using a three-dimensional cartilage spheroid model. In the Chan Lab, he will investigate the curious phenomenon of follicle growth competition and collective dynamics from a mechanobioloigical perspective.

Jake Turley
During his PhD at Univ. of Bristol, UK, Jake combined advanced microscopy, theoretical modelling, and machine learning to study epithelial cell dynamics during fly development and wound healing.
As a Schmidt AI In Science Postdoctoral Fellow in the Chan lab, he will continue to apply novel deep learning approach and biophysics to investigate mammalian ovulation during development and ageing. Learn more

Tan Jue Yu Kelly
Kelly joins the Chan Lab as a PhD student after obtaining her B.Sc. in Life Sciences from the National University of Singapore where she studied the mechanisms required for neuronal pruning in Drosophila. In the Chan Lab, she will explore how somatic cell dynamics and mechanosensing contribute to follicle morphogenesis. Learn more
Chan Lab Alumni
• Xixun Lu (SPS, Jan-June 2025)
• Le Mai Tan Dat (Daniel) (UROPS, Aug-Dec 2024)
• Lok Jie Bin (Summer Intern, Jun-Jul 2024)
• Manasvini Subramanian (Summer Intern, Jun-Jul 2024) — Current: PhD @ Ohio State University
• Teo Xin Ping (Summer Intern, Jun-Jul 2024)
• Nikita (Summer Intern, Jun-Jul 2024)
• Lee Chin Hao (FYP 2023-2024)
• Lou Yuting (Research Fellow, Apr 2023-Mar 2024) — Current: PI @ Fudan University, Shanghai
• Anna Jaeschke (Research Fellow, Oct 2021-Feb 2024)
• Aryan Mohanani (Summer Intern, Jun-Jul 2023)
• Kitty Anh Le (Summer Intern, Jun-Jul 2023) — Current: PhD @ MBI NUS
• Rothswell Montegrai Anak Lanting (Summer Intern, Jun-Jul 2023)
• Sukhada Darpe (Intern, Oct 2022-Apr 2023) — Current: PhD @ Weizmann Institute
• Vinod S/O Prabhakaran (Research Assistant, Aug 2021-Oct 2022) — Current: PhD @ MBI NUS
• Hugo Raveton (Master Intern, Jan-Jul 2022) — Current: PhD @ IBDM, Marseille
• Nafeesah Binte Mohamed Ibrahim (Summer Intern, Jun-Jul 2022) — Current: Masters @ ETH Zurich
• Santana Balaji (Summer Intern, Jun-Jul 2022)
• Lu Zhiyi (Summer Intern, Jun-Jul 2022)
• Wan Jun Gan (Research Fellow, Feb 2021-Feb 2022) — Current: Postdoc @ IMB, Univ. Queensland
• Wu Zihao (Research Assistant, Jun-Dec 2021) — Current: DDS @ Univ. Melbourne
• Nicole Loh Kit Ying (Summer Intern, Jun-Jul 2021)

About Chii Jou (Joe) Chan
Chii Jou (Joe) Chan was trained in theoretical soft matter physics at the University of Cambridge (B.A., M.Phil.). For his Ph.D. with Prof. Jochen Guck at Cambridge and TU Dresden (Germany), he studied the mechanical and optical properties of living cells and nuclei, using biomechanical tools (optical stretcher, microfluidics) and biophotonics. Inspired by how physical forces shape early development of living organisms, he joined the group of Dr. Takashi Hiiragi as an EIPOD fellow at EMBL Heidelberg (Germany), where he made a major discovery in hydraulic regulation of mouse embryo size and cell fate specification. His interdisciplinary productivity is reflected in the diversity of his collaborators (cell and developmental biologists, experimental biophysicists, theorists) across the world. Learn more
CHII JOU (JOE) CHAN LABORATORY
Recent publications
- Wohland T., Saunders T.E., Chan CJ. Developmental biophysics. Biophysical Journal. (2025)
- Turley J, Leong KW, Chan CJ. Novel imaging and biophysical approaches to study tissue hydraulics in mammalian folliculogenesis. Biophysical Reviews. (2024)
- Jaeschke A, Hepburn MS, Mowla A, Kennedy BF, Chan CJ. Three-dimensional quantitative micro-elastography reveals alterations in spatial elasticity patterns of follicles and corpora lutea in murine ovaries during ageing. bioRxiv. (2024)
- Biswas A, Lou YT, Ng BH, Tomida K, Darpe S, Wu Z, Lu TB, Bonne I, Chan CJ. Theca cell mechanics and tissue pressure regulate mammalian ovarian folliculogenesis. bioRxiv. (2024)
- Hepburn M, Jaeschke A, Mowla A, Chan CJ, Kennedy BF. Three-dimensional characterization of murine ovary elasticity using quantitative micro-elastography. Optical Elastography and Tissue Biomechanics XI. (2024), PC128440B
- Bevilacqua C, Gomez JM, Fiuza U-M, Chan CJ, Wang L, Hambura S, Eguren M, Ellenberg J, Diz-Muñoz A, Leptin M, Prevedel R. High-resolution line-scan Brillouin microscopy for live-imaging of mechanical properties during embryo development. Nature Methods. (2023)
- Biswas A, Ng BH, Prabhakaran V, Chan CJ. Squeezing the eggs to grow: The mechanobiology of mammalian folliculogenesis. Front. Cell Dev. Biol. (2022)
- Chan CJ, Hirashima T. Tissue Hydraulics in Reproduction. Seminars in Cell & Developmental Biology (2022)
- Chan CJ, Bevilacqua C, Prevedel R. Mechanical mapping of mammalian follicle development using Brillouin microscopy. Comm. Biology (2021) 4 (1133)
- Yang Q, Xue S-L, Chan CJ, Rempfler M, Vischi D, Gutierrez F M, Hiiragi T, Hannezo E, Liberali. Cell fate coordinates mechano-osmotic forces in intestinal crypt morphogenesis. Nature Cell Biology (2021).
- Roffay C*, Chan CJ*, Guirao B, Hiiragi T, Graner F. Inferring cell junction tension and pressure from cell geometry. Development (2021) 148 (18) dev192773.
- Bevilacqua C, Hambura S, Wang L, Chan CJ, Eguren M, Gomez Elliff JM, Diz-Muñoz A, Prevedel R. High-resolution line-scanning Brillouin microscopy for fast and low phototoxicity live-imaging of mechanical properties in biology. Elastography and Tissue Biomechanics VIII (2021), 11645
- Chan CJ, Hiiragi T. Integration of luminal pressure and signalling in tissue self-organisation. Development (2020) 147 (5), 1-10
- Ryan AQ, Chan CJ, Graner F, Hiiragi T. Lumen expansion facilitates epiblast-primitive endoderm fate specification during mouse blastocyst formation. Developmental Cell (2019) 51, 1-14.
- Chan CJ, Costanzo M, Ruiz-Herrero T, Mönke G, Petrie R, Bergert M, Diz-Munoz A, Mahadevan L, Hiiragi T. Hydraulic control of mammalian embryo size and cell fate. Nature (2019) 571:112-116
- Chan CJ, Heisenberg C-P, Hiiragi T. Coordination of morphogenesis and cell fate specification in development. Current Biology. (2017) 27(18):R1024-R1035.
- Chan CJ, Hiiragi T. Keeping in touch to differentiate. Developmental Cell (2017) 43(2):113-114
- Chan CJ, Li W, Cojoc G, Guck J, Volume transitions of isolated cell nuclei induced by rapid temperature increase. Biophysical Journal (2017) 112(6):1063-1076
- Schürmann M, Scholze J, Müller P, Guck J, Chan CJ. Cell nuclei have lower refractive index and mass density than cytoplasm. Journal of Biophotonics (2016) 9(10): 1068-1076.
- Chan CJ, Ekpenyong AE, Golfier S, Li W, Chalut KJ, Otto O, Elgeti J, Guck J, Lautenschläger F. Myosin II activity softens cells in suspension. Biophysical Journal (2015) 108(8): 1856–1869
- Schürmann M, Scholze J, Müller P, Chan CJ, Ekpenyong AE, Chalut KJ, Guck J. Refractive index measurements of single, spherical cells using digital holographic microscopy. Methods in Cell Biology (2015) 125:143-159.
- Chan CJ, Whyte G, Boyde L, Salbreux G, Guck J. Impact of heating on passive and active biomechanics of suspended cells. Interface Focus (2014) 4, 20130069.
- Chalut KJ, Höpfler M, Lautenschläger F, Boyde L, Chan CJ, Ekpenyong AE, Martinez-Arias A, Guck J. Chromatin decondensation and nuclear softening accompany Nanog downregulation in embryonic stem cells. Biophysical Journal (2012) 103(10): 2060-2070.
- Chan CJ, Terentjev EM. Non-equilibrium statistical mechanics of liquid crystals: relaxation, viscosity and elasticity. Journal of Physics A (2007) 40 R103-R148 Topic Review.
- Chan CJ, Terentjev EM. Non-equilibrium statistical mechanics of nematic liquids. IMA Volumes in Mathematics and Its Applications, Modeling of Soft Matter (2005) 141:27-84.
CHII JOU (JOE) CHAN LABORATORY
Outreach
Lab Meeting with the Chan Lab – the Node

Bridging physics and biology
I have a long-standing passion towards bridging and fostering collaborations between biologists and physicists. Here are some great articles I love to share:
- Physical and mathematical modeling in experimental papers
- After the Greeting: Realizing the potential of physical models in cell biology
- Theory in Biology: Figure 1 or Figure 7?
- Experimentalist meets Theoretician: A tale of two scientific cultures
MBI Theory Journal Club
(05/05/21): I am excited to share my perspectives on the physics of tissue hydraulics.
Chan Lab manual [MBI personnel only]
Getting to Sit on the Other Side of the Desk in Academia
MBI SPSS series (17/11/2020): I have the privilege to share my experience on my journey towards becoming a PI.
Joe hosts students from Raffles Girls’ School at MBI
Joe giving a talk at Raffles Girls’ School
Joe shares latest research with > 100 students from Anglo Chinese Junior College.



The Chan lab had great fun sharing with the younger generation the joy of scientific discovery!
On March 18, 2022, MBI hosted a visit from students from NUS High School. One of their highlights was a lab tour, where the Chan lab demonstrated their stereomicroscope and how they use it for their research.

 2019 Lautenschlager Summer School Lecture
2019 Lautenschlager Summer School Lecture
EMBL Heidelberg (18/7/2019): I am privileged to give a lecture on the (Bio)physics of early embryo morphogenesis.
 Embryonic hydraulics triumphs
Embryonic hydraulics triumphs
Here’s a story about our research in the Node with wonderful collaborators like Ryan Petrie!

CONTACT US
Chii Jou (Joe) Chan Laboratory
Chii Jou (Joe) Chan
Principal Investigator, Mechanobiology Institute,
National University of Singapore
Mechanobiology Institute
National University of Singapore
T-Lab, #10-01
5A Engineering Drive 1
Singapore 11273
Email: dbschii@nus.edu.sg
Phone: +65 6601 1554 Ext. 11554
Web: www.mbi.nus.edu.sg/chii-jou-chan/











 2019 Lautenschlager Summer School Lecture
2019 Lautenschlager Summer School Lecture

