The Pressure That Shapes Life: Mechanical Forces Behind Egg Maturation
First quantitative characterisation of mechanical stress within mammalian follicles reframes the role of theca cells in maintaining follicle integrity.
Researchers from the Chan Lab at MBI demonstrate the importance of compressive stress exhibited by theca cells in healthy follicle maturation and surrounding support cells.
The maturation of functional eggs within ovarian follicles is essential for successful reproduction in mammals. While mechanical signaling has been suggested as a factor in successful folliculogenesis, little is known about the forces within the follicles that influence their development and function.
A new study led by Arikta Biswas from the Mammalian Development and Tissue Hydraulics Laboratory (Chan group) at the Mechanobiology Institute, NUS, has uncovered that a layer of cells encasing developing follicles, known as theca cells, actively squeeze the follicles through contractile forces. The findings published in Nature Communications show that this compression is vital to the healthy growth of eggs and their surrounding support cells.
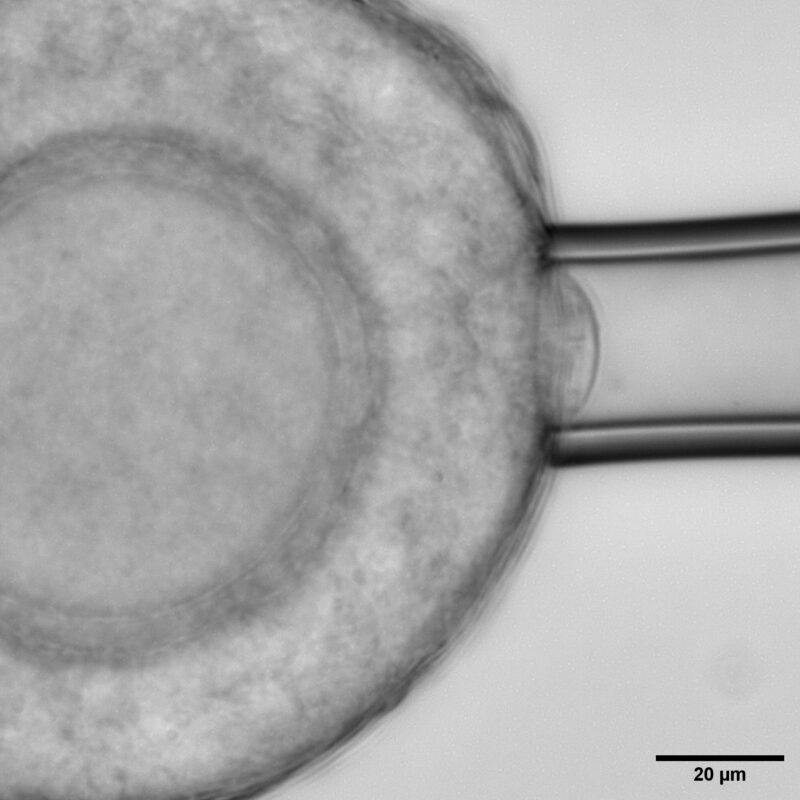
Micropipette aspiration on the follicle surface to measure effective follicle surface tension.
Using young mouse ovaries as a model, the researchers found that theca cells, are far from passive. These spindle-shaped cells at the follicle’s outer boundary are highly contractile, generating measurable compressive stress that regulates the follicle’s internal mechanics. By combining biophysical approaches like atomic force microscopy, micropipette aspiration, and laser ablation, with perturbations and live imaging; the team quantified changes in theca cell and tissue mechanics that proved to be important for regulating follicle morphogenesis.
When the researchers reduced theca cell contractility using chemical inhibitors, the follicles swelled and lost their compressive stress, leading to a drop in the internal pressure and suggesting that theca cells act as a constrictive shell maintaining the follicle’s compact form. Enhancing contractility produced the opposite effect, tightening the tissue and increasing pressure. These experiments revealed that the developing follicle is not a passive structure but a mechanically active system whose growth depends on internal and external forces held in balance.
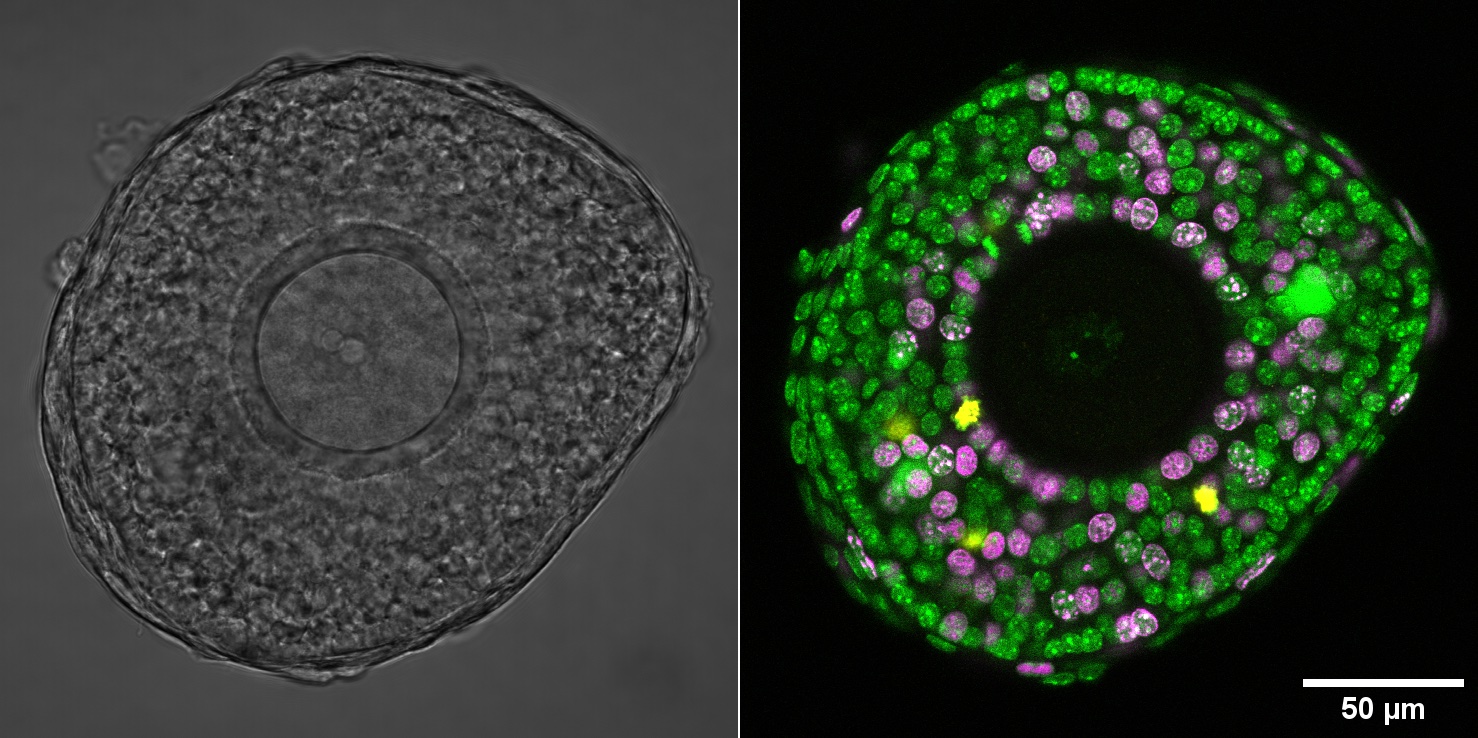
Secondary follicle showing spatial patterns of cell-fate and functions. Green: All cell nucleus; Magenta: Nucleus in S-phase of cell cycle; Yellow: Nucleus in M-phase cell cycle
This mechanical stress also governs how cells inside the follicle behave. The study found that granulosa cells respond almost immediately to compressive stress via the YAP mechanosensitive signaling pathway. The researchers also observed that physical changes disrupted transzonal projections, the fine bridges that connect granulosa cells to the oocyte, suggesting that follicles can sense and respond to mechanical cues on remarkably short timescales.
Over longer periods, sustained disturbances in compressive stress slowed follicle growth and caused sweeping changes in gene expression within granulosa cells. Genes related to inflammation, extracellular matrix remodeling, and apoptosis became more active, while those promoting growth were suppressed. The oocytes themselves remained comparatively stable, hinting that the surrounding tissue acts as a mechanical buffer protecting the egg. Together, these findings point to the existence of an optimal level of compression necessary for healthy follicle development—too much or too little, and the process falters.
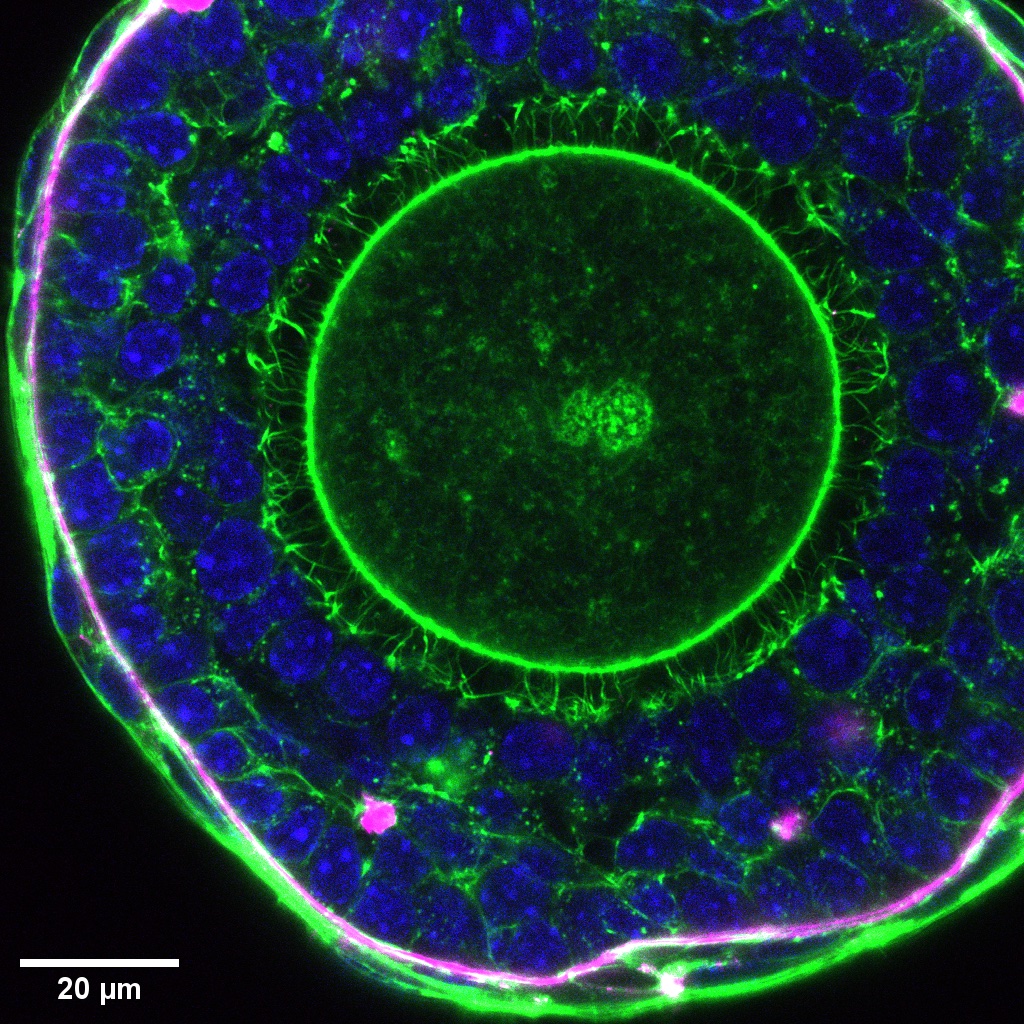
- High resolution image of a secondary follicle showing nucleus (blue), basement membrane (magenta), and actin at cell boundary (green). Transzonal projections can be seen around the oocyte.
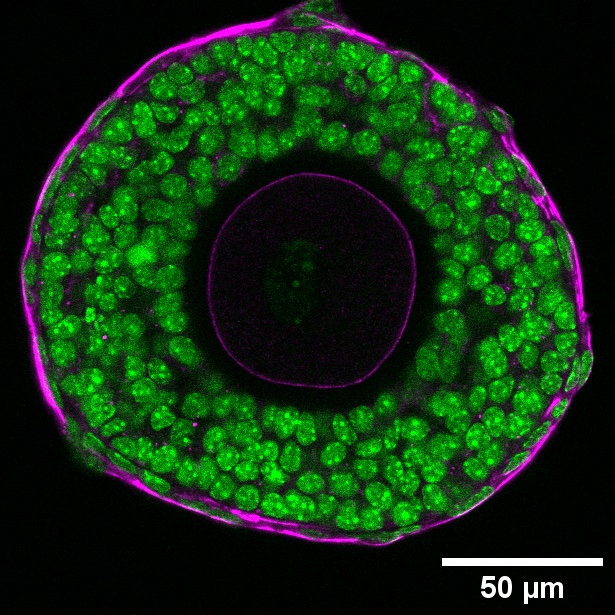
- The cells (nucleus, green) surrounding the developing follicles are contractile (phosphorylated myosin light chain, magenta).
By offering the first quantitative characterisation of mechanical stress within mammalian follicles, the study reframes the role of theca cells beyond hormone production. It positions them as key mechanical regulators that maintain follicle integrity and pressure, orchestrating growth from the outside in. This has suggests a new perspective in understanding infertility and disorders such as polycystic ovary syndrome (PCOS), where abnormal theca cell behavior is common.
Beyond reproduction, the research reinforces a broader biological principle: that compressive stress is a universal regulator of growth, guiding everything from tissue development to disease progression. As Biswas and colleagues suggest, decoding these hidden physical forces could pave the way for new forms of “mechano-therapeutics”—approaches that manipulate tissue mechanics to improve egg maturation and fertility outcomes.