Signaling in 3D
LAKSHMI RAMACHANDRAN, PHD | MAY 2017
A collaborative study from the Mechanobiology Institute (MBI), National University of Singapore (NUS), and the FIRC Institute of Molecular Oncology (IFOM), Italy, has shown for the first time, how the shape of cells within tissues determines their response to biochemical signals. This study led by Aninda Mitra, IFOM postdoctoral fellow, together with Saradha Venkatachalapathy, MBI graduate student from the lab of Professor GV Shivashankar, Deputy Director of MBI, demonstrates the differential impact of two divergent cell shapes/geometries on gene regulation by TNFα, an immune regulatory protein that plays a role in cancer. This work is published in PNAS (Mitra A, Venkatachalapathy S, Ratna P, Wang Y, Jokhun DS, Shivashankar GV, Cell geometry dictates TNFα-induced genome response, Proc Natl Acad Sci U S A. 2017 May 1. pii: 201618007. doi: 10.1073/pnas.1618007114).
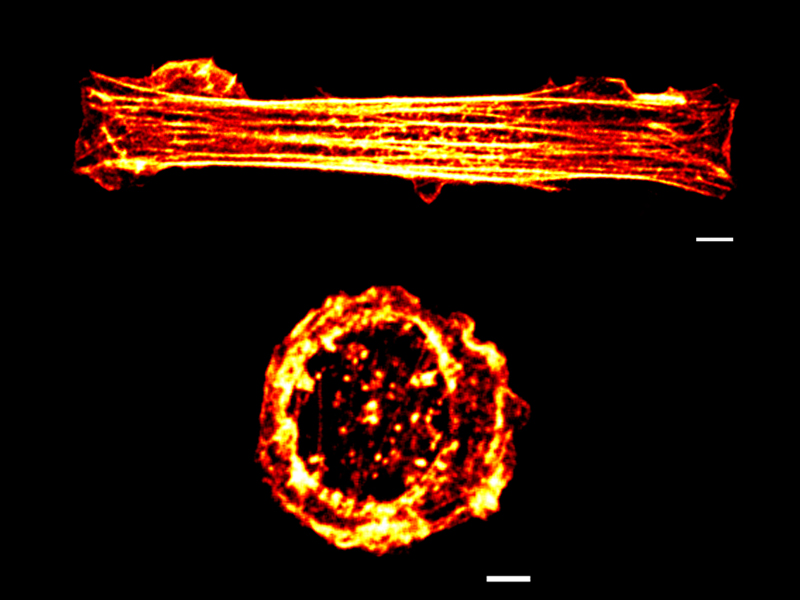
Rectangular stretched (top) and circular relaxed (bottom) cell geometries observed under confocal microscopy. The cells are stained with phalloidin, which stains actin, a component of the cytoskeleton. Note the well-organized filamentous cytoskeleton in the rectangular vs. circular cells. White, yellow and red colour indicates the flourescence intensities from high to low.
MBI Scientists reveal a spatial dimension to cell signaling
The cells that make up the human body are constantly responding to, and generating, biochemical and mechanical signals. These signals include hormones, growth factors, neurotransmitters, immune regulators (cytokines), as well as sensory signals. Whether these signals originate from mechanical (blood pressure or touch) or biochemical sources, they are nearly all transmitted via a series of biochemical reactions that converge on the cell nucleus. With the nucleus housing the cell’s genome, any signal that reaches it will impact which genes are turned on or off. This in turn initiates distinct cellular responses. The ability to specifically activate or deactivate genes is crucial for normal cell physiology and function, and any aberrations in cell signaling may lead to disease states such as fibrosis and cancer.
How does cell geometry regulate biochemical signaling?
Years of research into biochemical signaling has already provided researchers with a strong understanding of how gene programs are defined by specific biochemical pathways. Recent studies have also shown that mechanical properties of the extracellular matrix (ECM), which is a complex mesh of fibrous proteins and polysaccharides on which cells embed and attach, also regulate cell behavior. Despite the vast range of biochemical and mechanical signals already defined, it is becoming increasingly clear that cells also take on important information from external sources such as their immediate microenvironment.
With this in mind, researchers from the FIRC Institute of Molecular Oncology (IFOM), who are based at the Mechanobiology Institute, Singapore, sought to determine whether physical properties that define the cell as a whole, can influence the biochemical pathways that drive cell function.
The finding that the mechanical states of cells helps dictate their response to biochemical signals has far-reaching implications.
One property that was of particular interest to the team was cell geometry. That is, the shape that cells assume as they are organized into various tissues. Importantly, changes to cell shape are commonly observed in cancer. Cells found in healthy connective tissue, for example, have an elongated shape, yet these same cells become round as they turn cancerous. The rounding up of cancer cells is also associated with their detachment from the ECM and neighbouring cells.
It had recently been shown that cells can exhibit distinct gene expression patterns if their geometries were altered. This inspired the researchers to assess the influence of cell geometry on a biochemical signaling pathway that is commonly impaired in cancer; the tumour necrosis factor alpha (TNFα) pathway.
To achieve this Mitra et al used techniques to grow cells in the lab that mimicked two naturally occurring cell geometries – a rectangular, stretched shape, and a circular relaxed shape. The cells were then stimulated by adding the cytokine TNFα.
What was discovered was that the pattern of genes switched on or off was significantly different depending on whether the cell geometry was rectangular or circular. Importantly, the scientists demonstrated that these genetic differences are related to the varying states of cytoskeleton organization in the two cell geometries. The cytoskeleton is essentially a network of protein cables that provide structural support to the cell. These same filaments can also be assembled into structures that allow the cells to interact with both the surrounding matrix, and neighboring cells.
In rectangular cells, the cytoskeleton is robust and well-organized, and this is in contrast to circular cells. This structural disparity alone was sufficient for proteins that are controlled by TNFα to move into the nucleus of the rectangular cells, and bring about diverse patterns of gene expression.

Visiting Research Fellow Aninda Mitra in the MBI labs
The finding that the mechanical states of cells helps dictate their response to biochemical signals has far-reaching implications. By taking into account the three-dimensional organization of cells in tissues, this study closely mimics the situation cells naturally exist in, to clearly demonstrate how the TNFα biochemical signaling pathway is regulated by cell geometry. In this case, biochemical and mechanosignaling pathways are mutually inclusive. These fresh insights into how the altered mechanical states affects genome regulation in tumors also shed light on the pathophysiology of cancer, and open up new avenues to investigate the cause of this disease.