Quantitative Micro-Elastography: New Imaging Tool Uncovers Age-Dependent Mechanical Changes in Ovarian Tissue
Detailed 3D maps of ovarian stiffness in mice reveal crucial insights that could lead to new infertility treatments.
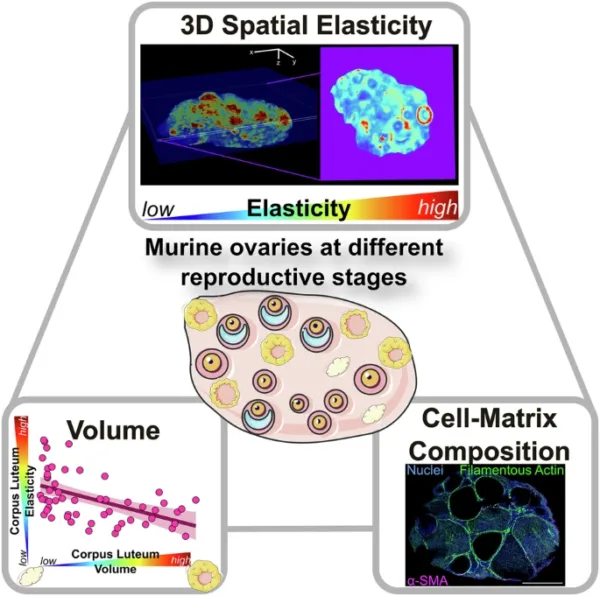
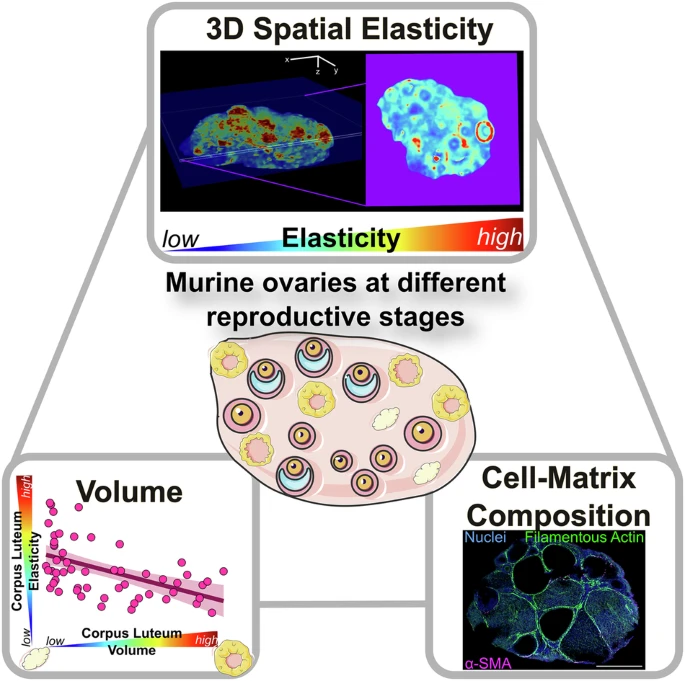
Researchers from the Chan Lab at MBI demonstrate a proof-of-principle approach to generate multidimensional tissue information, linking tissue elasticity to the morphology and cell-matrix composition of tissue architecture.
Researchers at the Mechanobiology Institute (MBI), National University of Singapore have developed an innovative imaging approach that offers the clearest picture yet of how the mechanical properties of ovarian tissue change with age. The study published in Communications Biology was led by MBI Research Fellow Dr. Anna Jaeschke and Assistant Professor Chii Jou Chan, in collaboration with Dr. Matt Hepburn and Prof. Brendan Kennedy from the University of Western Australia, used a novel method called quantitative micro-elastography to create detailed three-dimensional maps of ovarian stiffness in mice—revealing crucial insights that could lead to new infertility treatments.
Fertility in women begins to decline significantly in their late 30s and early 40s, largely due to a reduction in both the quality and quantity of eggs. Most fertility research and treatments have traditionally focused on correcting hormonal imbalances associated with aging. However, in recent years, scientists have turned their attention to the structural and mechanical aspects of the ovaries, seeking to understand how the tissue itself changes over time and contributes to fertility loss.
Earlier studies using techniques like indentation microscopy suggested that ovarian tissue becomes stiffer with age, a finding linked to infertility. But those methods could only provide a broad view at the organ level, without capturing how individual parts of the ovary—such as follicles, the corpus luteum, and the surrounding matrix—change dynamically during aging. These components each play specific and critical roles in the reproductive process, and understanding their unique mechanical properties is key to developing more effective fertility treatments.
- Quantitative micro-elastography (QME), a label-free, non-invasive method to study 3D microscale elasticity in conjunction with immunofluorescence microscopy. Image credit: https://doi.org/10.1038/s42003-025-08835-w
The new imaging technique used in this study overcomes the limitations of previous methods. Unlike many indentation-based approaches, which were invasive and could alter the tissue mechanical properties during sample preparation, quantitative micro-elastography allows researchers to measure ovarian tissue mechanics in three dimensions with minimal damage. It also offers deep tissue penetration, faster scanning speeds, and the ability to resolve features down to 35 micrometres, enabling tissue-scale mechanical mapping across the entire ovary.
To investigate how tissue stiffness evolves with age, the team examined ovaries from mice at three life stages—3 weeks, 9 weeks, and 12 months old. By combining their elasticity maps with immunofluorescence imaging, they were able to correlate stiffness patterns with tissue volume and biochemical composition, including changes in collagen levels within the extracellular matrix.
While earlier studies suggested that tissue stiffness increased uniformly from the inner to the outer regions of the ovary, this study revealed a much more complex and heterogeneous distribution. One of the surprising discoveries was the role of the corpus luteum—a hormone-secreting structure formed after ovulation—in driving this variability. The stiffness of the corpus luteum increased with age and fluctuated depending on the stage of its development, suggesting that it is a key dynamic contributor to shape the ovary’s mechano-microenvironment during ovulatory cycles and ageing.
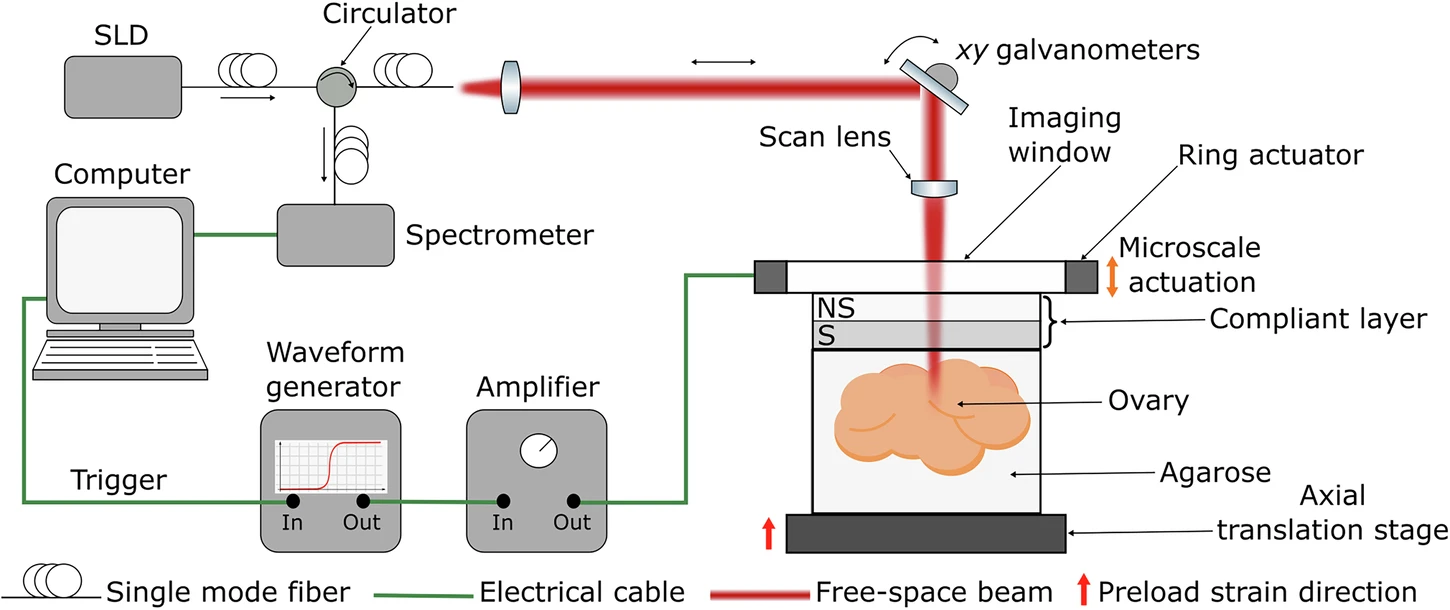
Schematic representation of QME setup. Image credit: https://doi.org/10.1038/s42003-025-08835-w
The researchers also uncovered age-related changes in the composition of the extracellular matrix in the corpora lutea. Levels of collagen-I and collagen-III, two important structural proteins, changed during ageing, indicating significant tissue remodelling during the growth and regression of the corpus luteum. These changes in stiffness and matrix composition appear to be closely linked and may play an underappreciated role in reproductive aging.
In contrast, the egg-carrying follicles showed relatively stable elasticity throughout the aging process. However, within larger follicles, there were notable differences in stiffness between regions. The outer layer, known as the theca cell layer, was found to be stiffer than the inner regions—a structural distinction the researchers believe could have a protective role during ovulation.
Together, these findings demonstrate the power of quantitative micro-elastography to reveal fine-scale changes in ovarian tissues that were previously understudied. By identifying how specific structures like the corpus luteum and follicular layers change with age, the study opens new doors for fertility research.
Future therapies to target the mechanical properties of ovarian tissue—not just hormonal levels—may offer a complementary approach to treat infertility during ageing. In addition, this work demonstrates a proof-of-principle approach to generate multidimensional tissue information, linking tissue elasticity to the morphology and cell-matrix composition of tissue architecture. Such methods may be adapted for other tissue mechanobiological research in development, health, and disease.