Protecting the Genome
Steven J Wolf | July 2014
A collaborative study between the FIRC Institute of Molecular Oncology (IFOM), the University of Milan, the Mechanobiology Institute, Singapore (MBI), and the Danish Cancer Society Research Center has revealed that ATR, a protein known to prevent DNA damage, is activated by mechanical signals. This work was published in Cell (Kumar A, Mazzanti M, Mistrik M et al. ATR Mediates a Checkpoint at the Nuclear Envelope in Response to Mechanical Stress. Cell, 2014; 158(3):633-646).
ATR responds to mechanical stimuli to protect DNA
Prevention is better than cure. This phrase rings true in many scenarios, but none more so than in cancer. While we strive to prevent the onset of the disease by minimizing our exposure to known carcinogens, we have little control over DNA damage, or the introduction of mutations into our genome; yet this is where cancer originates. Fortunately, our cells also live by this principal, and have evolved intrinsic mechanisms that protect DNA from the introduction of mutations, and repair it when it is damaged.
Protecting the genome from mechanical stress
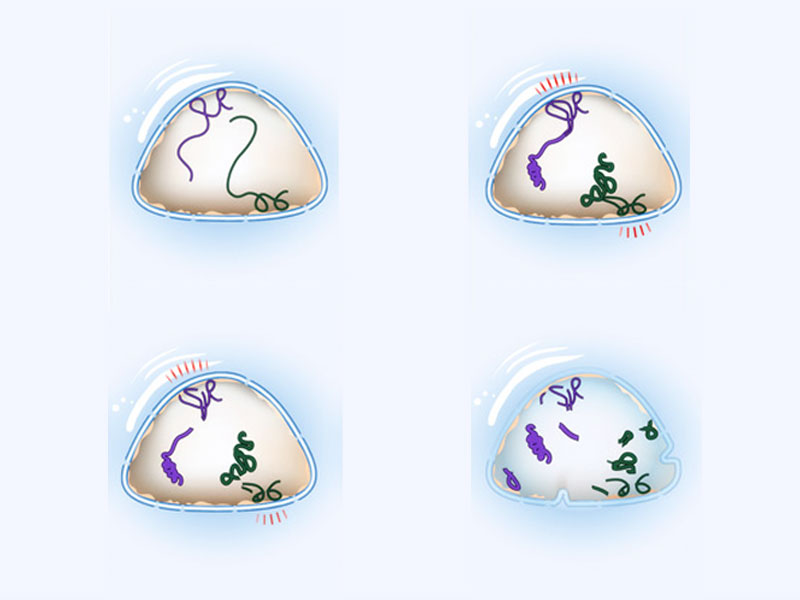
For much of a cell’s life, DNA exists as a highly condensed ‘wound up’ structure called chromatin. When it is time to replicate or decode the DNA, chromatin is unwound and the various proteins that process DNA are able to move along the molecule with unhindered access to the genetic code. However, certain regions of the chromatin are attached to the inside of the nuclear membrane and this can pose problems during both condensation and DNA replication. In the latter, the replication machinery may be hindered, and the DNA copied inaccurately. Such obstacles must be overcome to prevent abnormal chromatin dynamics and the introduction of genetic anomalies.
To fully understand how mechanical forces confer a biological response, a multidisciplinary approach to the research is required.
Understanding how cells pre-empt collisions between the replication machinery and regions of chromatin still bound to the nuclear membrane was the focus of a collaborative study published in Cell and led by Prof. Marco Foiani of IFOM, Italy. In this study a protein known as ATR, which senses DNA damage and protects genome integrity, was revealed to respond to mechanical stimuli originating at the nuclear envelope. Here, ATR rapidly moved to the nuclear envelope when the nucleus was stretched by changes in osmotic pressure, or when whole cells were stretched via a patch-clamp method. Once present at this site, activation of the ATR signalling pathway was found to co-ordinate chromatin condensation and nuclear envelope breakdown, which enables detachment of condensed chromatin from the nuclear envelope. Such processes ensure chromatin condensation proceeds normally and DNA replication progresses unhindered.
While ATR’s role in the prevention of DNA damage was well known, the role of mechanical forces in its regulation took the researchers by surprise. It also took them into unfamiliar territory, where a fundamental biological process was regulated by basic physics. A deeper understanding of the mechanisms involved was however attained through collaboration with Prof. G.V. Shivashankar at the Mechanobiology Institute, National University of Singapore.
In healthy cells, torsional stress generated within DNA is imposed as mechanical force on the nuclear membrane. Additional forces are conferred to the nucleus when cells are stretched or compressed by neighbouring cells, or during processes such as cell motility. In the latter cases, these forces are transduced to the nucleus via a network of filaments known as the cytoskeleton.
Traditionally, researchers have focused on the role of biochemical pathways and genetics in understanding how cells protect the genome and repair damaged DNA. However, the findings published by Kumar et al, highlight the importance of mechanical force in the regulation of fundamental biological processes. In this case, aberrant chromatin dynamics, and DNA damage, which can potentially introduce cancer causing mutations into the genome, is avoided when ATR is activated by a mechanical signal.
To fully understand how mechanical forces confer a biological response, a multidisciplinary approach to the research is required. In 2014, collaborations between IFOM and MBI were cemented through the formation of the Joint Research Laboratory and chaired professorship. This relationship will continue to promote a deeper understanding of how physical forces modulate biochemical processes, and in turn, reveal new discoveries in the etiology of cancer.
IFOM-MBI collaborations strengthened
This work was presented by Prof. Marco Foiani at a recent mini-symposium hosted by MBI.
The Mechanobiology Institute, National University of Singapore (MBI) has inked a Memorandum of Understanding (MoU) with the FIRC Institute of Oncology, Italy (IFOM) to establish a Joint Research Laboratory based at the MBI. For an initial duration of 3 years, the Joint Research Lab will be embarking on a project on the “Mechanobiology of Cancer”.
The Joint Research Lab will be headed by Prof. G V Shivashankar, Deputy Director of the MBI with his counterpart Prof. Marco Foiani, Scientific Director of IFOM. Alongside the Joint Research Lab, the partnership was marked with the creation of an IFOM-NUS Chaired Professorship held by Prof Shivashankar.
For more information visit: http://www.ifom.eu/en/press-area/news-press-releases/2014.php?docuID=3346