Microfluidics for Liquid Biopsies
Sruthi Jagannathan | January 2016
Scientists from the National University of Singapore (NUS) have developed a miniaturized device that can rapidly and efficiently detect and isolate cancer cells from whole blood. Based on an emerging technology called microfluidics, the device adopts a simple, low-cost and a highly non-invasive methodology and provides an ideal platform for point of care cancer diagnostics and treatment. This work is led by Prof Lim Chwee Teck, who is also a Principal Investigator at the Mechanobiology Institute (MBI), NUS.
A Tool for Point-of-Care Cancer Diagnostics and Treatment
Microfluidics is characterized by the precise manipulation of fluid within sub-millimeter channels, at which scale the effect of physical properties, such as capillary force and surface tension, on fluid behavior is considerably greater than at the macro-scale. Rapid advancements in the technology has expanded biomedical research through the development of lab-on-a-chip devices for a number of biochemical analyses such as cell separation, DNA and protein analyses, PCR amplification, and so on.
By applying microfluidics to the detection and isolation of cancer cells, the team of researchers developed a spiral microfluidic chip that enables an ultrafast and efficient, size-based separation and isolation of circulating tumor cells (CTCs) from whole blood. As the name implies, circulating tumor cells are cancer cells that get disseminated in the peripheral bloodstream, and over the course of the disease, invade other body parts, where they proliferate to form secondary tumors. The ability of cancer cells to spread aggressively is known as metastasis and is responsible for the majority of cancer- related deaths.
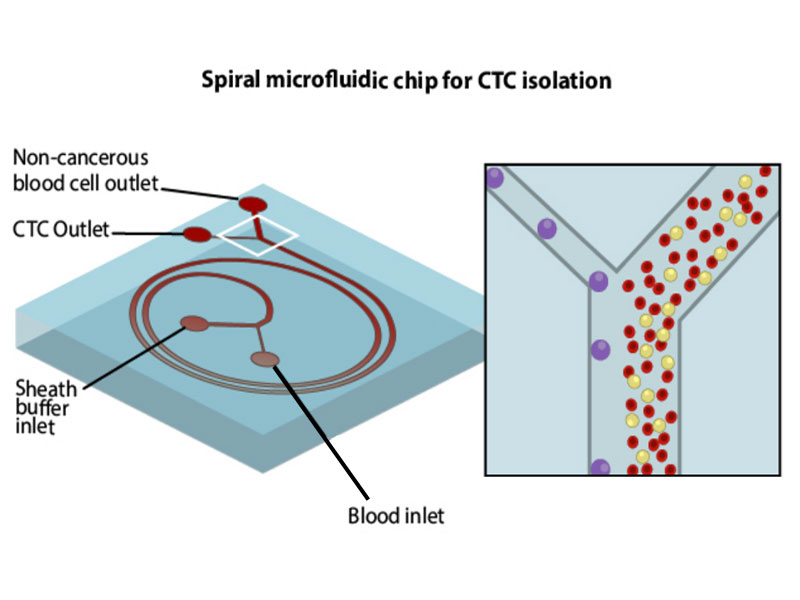
A spiral microfluidic chip for isolating cancer cells
CTCs are extremely rare cells that start occurring in the early stages of cancer, and their levels rise steadily as the disease progresses. Therefore, unlike tissue biopsies that can be used only as a diagnostic tool and can only be performed once before and after cancer treatment, liquid biopsies involving the isolation and enumeration of CTCs can be used for real-time diagnosis as well as for assessing metastatic risk, prognosis and treatment efficacy in patients during treatment. Conventional cell separation methods developed so far for the isolation of CTCs have been limited by disadvantages such as poor CTC detection sensitivity, high degree of damage to isolated CTCs or contamination with other blood cells, and their inability to process large volumes of blood.
The spiral microfluidic chip offers many advantages over other cell separation systems, such as portability, the need for low volumes of sample and ultra-fast processing time.
The spiral microfluidic chip described in this study successfully overcomes the challenges involved in the isolation and characterization of such rare blood cells. The chip is made of a silicon-based organic polymer and consists of two loops of micro- channels that are etched using a patterning technique called soft lithography. Once the blood is pumped through the inlet at an optimized flow rate, the spiral design and the dimensions of the channels create hydrodynamic forces that cause the larger CTCs to flow along the inner wall of the channel, whereas the smaller, non-cancerous blood cells flow along the outer wall. By collecting the separated blood cell fractions via different outlets, the technique ensures negligible contamination of CTCs with other non-cancerous blood cells, and an almost 100% CTC detection sensitivity. The device also has the ability to continuously process blood samples, leading to a high recovery of CTCs. The small size of the microfluidic device allows multiple units to be stacked together that can further increase the diagnostic output.
Beside their economic feasibility and enhanced performance, the spiral microfluidic chip offers additional advantages over other cell separation systems, such as portability, the need for low volumes of sample and ultra-fast processing time. The methodology thus has great potential to be translated into a highly reliable and patient- friendly clinical test for the diagnosis, prognosis, management and treatment of cancers. The recovery of highly pure fractions of CTCs also enables its use for downstream DNA and protein analyses, which can reveal vital information on cancer biology and serve as research platforms for the discovery of new cancer drugs and their pre-clinical testing.
A detailed protocol describing the principle of operation, design, fabrication and applications of the spiral microfluidic chip has been recently published in Nature Protocols [Warkiani ME et al. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nature Protocols. January 2016. 11(1)].