Epithelial Tube Contraction
Andrew MS Wong, Animation by Cindy Zhang | December 2014
Researchers at the Mechanobiology Institute (MBI), National University of Singapore have identified a novel mechanosensitive regulation of epithelial tube contraction. These findings are published today in Current Biology (Pei Yi Tan and Ronen Zaidel-Bar. Transient membrane localization of SPV-1 drives cyclical actomyosin contractions in the C. elegans spermatheca, Current Biology, 19 Dec 2014, doi: 10.1016/j.cub.2014.11.033).
A new feedback mechanism for regulating contractility
Many of the fundamental processes of life rely on biological structures known as epithelial tubes. These tubes serve to transport various gases, liquids and cells around the body. With each breath, for example, epithelial tubes transport oxygen to the lungs. Our blood vessels, kidneys and pancreas, mammary, salivary and tear glands, are all essentially composed of epithelial tubes. However, these tubes are more than just a biological plumbing system. Instead, they are dynamic structures which must counter outward pressures to prevent their swelling or rupture. An intrinsic ability of epithelial tubes to constrict helps maintain their integrity. This constriction results from actomyosin contractility, a co-ordinated movement of filaments made of a protein known as actin, and a motor protein known as myosin. Problems in epithelial tube contractility are responsible for asthma, raised blood pressure and gastrointestinal disorders. Each one of these diseases affects millions of people worldwide, dramatically affecting their quality of life.
Regulating tube constriction
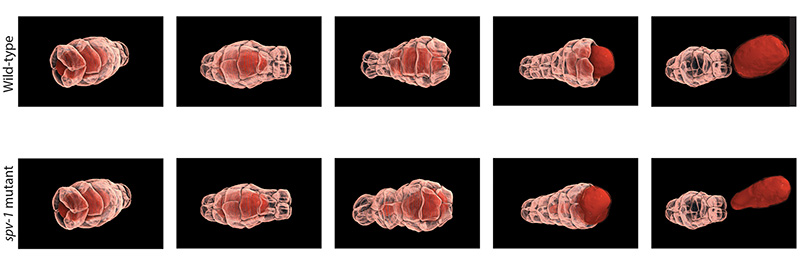
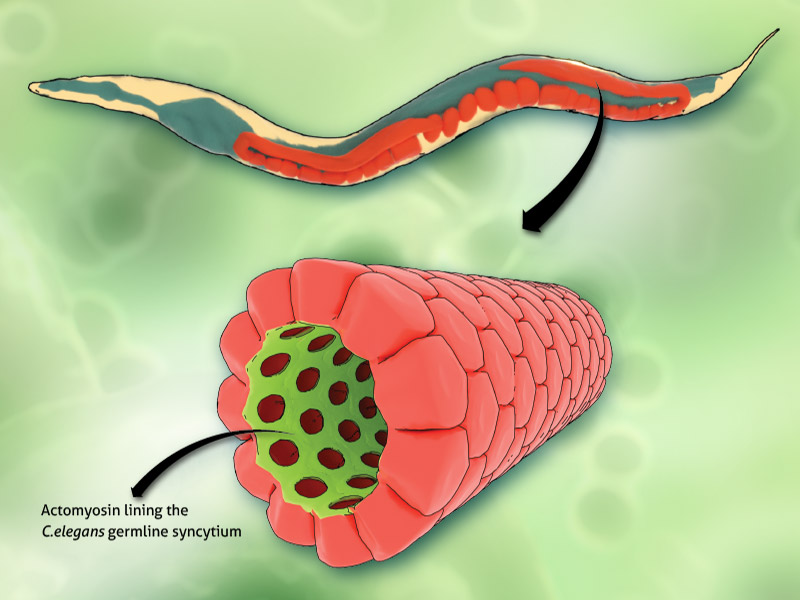
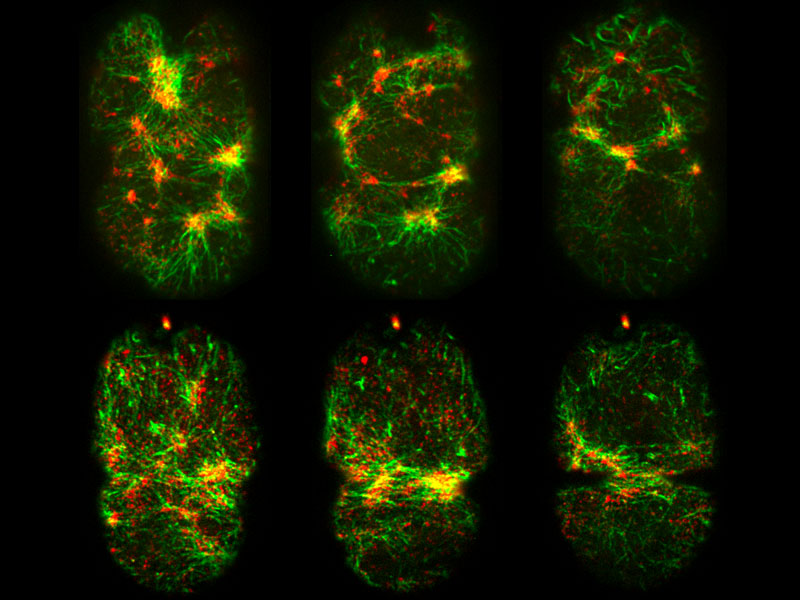
MBI scientists have discovered a feedback mechanism that regulates epithelial tube contractility in a living organism, the C. elegans nematode worm. C. elegans is a hermaphrodite, and can therefore reproduce by self-fertilization. Within the worm is a ‘conveyor belt’ of unfertilised eggs. During ovulation, a single egg is released into a structure known as the ‘spermatheca’, which is an accordion shaped tube that houses the sperm. Following fertilisation, an actomyosin based contraction of the tube expels the fertilised embryo into the uterus. This cycle is repeated approximately 150 times throughout the lifetime of the adult worm.
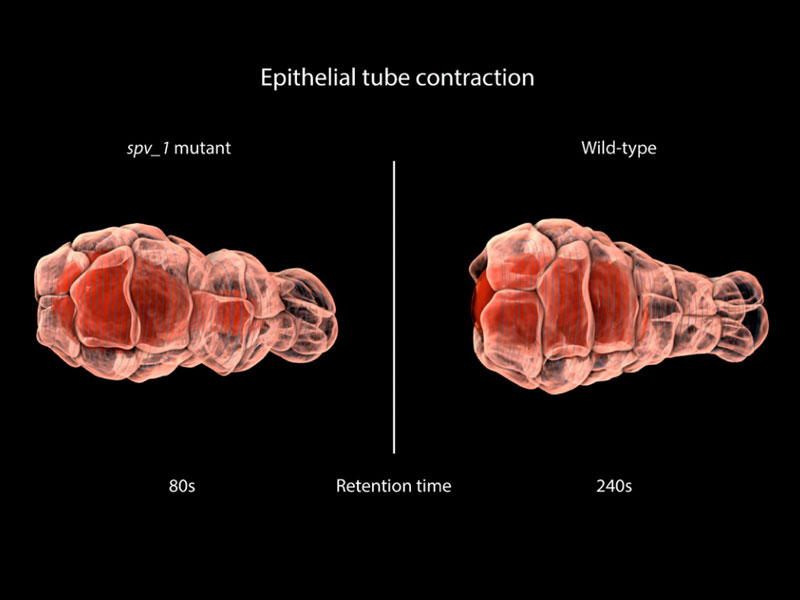
This contraction is similar to that observed in other epithelial tubes. The similarities led MBI Principal Investigator, A/Prof. Ronen Zaidel-Bar and graduate student Pei Yi Tan to use the spermatheca as a model to study epithelial tube contraction. In doing so, a novel mechanism of regulation was identified, and a protein known as SPV-1 shown to maintain regular cycles of actomyosin contractility. In mutants lacking SPV-1 function, a hyperactive contractility was noted, with embryos propelled from the spermatheca prematurely.
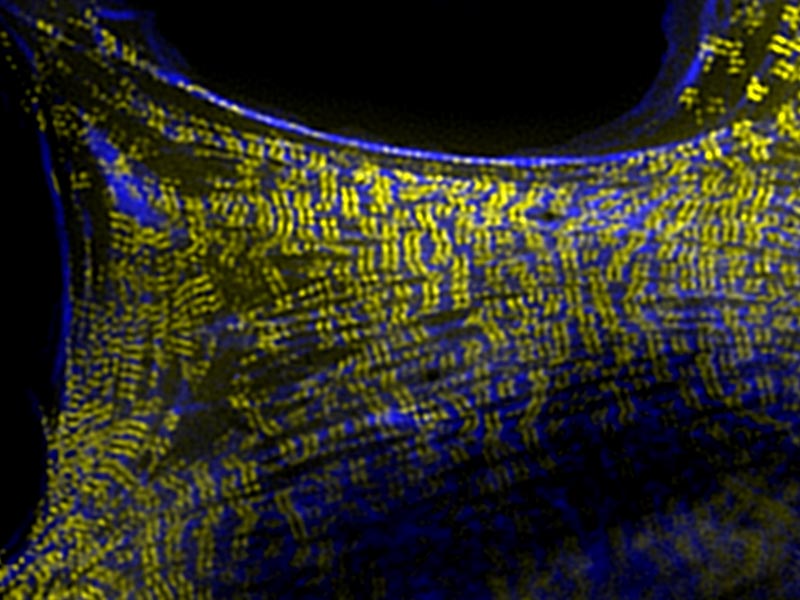
Remarkably, it was the interplay between SPV-1 localization within the cell, and the shape of the cell membrane, that controlled tube contractility. In an empty spermatheca, SPV-1 attached to the folded, convoluted cell membranes where it acted as a protein roadblock preventing actomyosin contractility. However, as the spermathecal cells stretched upon entry of the egg, SPV-1 detached from the straightened membrane. With the roadblock removed, actomyosin contractility occured and a fertilised embryo was ejected into the uterus. Once the reproductive cycle was completed, the spermatheca collapsed, and SPV-1 re-localized to the folded membrane. However, when SPV-1 was mutated, it remained attached to the membrane irrespective of membrane curvature. In this case the actomyosin failed to contract, leading to the accumulation of multiple embryos in the spermatheca. This finding confirmed that SPV-1 is indeed mechanosensitive.
The elegant feedback system discovered in this study links the regulation of contraction to the shape and function of epithelial tubes via mechanical and biochemical signalling. It may also represent a universal mechanism that regulates contractility in other epithelial tubes, including those found throughout our bodies. Extending this mechanism to our current knowledge of contractility and understanding how it regulates human epithelial tube constriction could lead to novel treatments for diseases such as asthma or cardiovascular disease.