Circulating Tumor Cells
Sruthi Jagannathan | May 2015
A collaborative study led by scientists from the Mechanobiology Institute, the Cancer Science Institute of Singapore and the National University Hospital at the National University of Singapore, along with scientists from the Singapore-MIT Alliance for Research and Technology (SMART) centre has led to the development of a novel technique for culturing circulating tumor cells (CTCs) that could be used to predict cancer treatment outcome. This study is published in Oncotarget (Khoo BL. et al, Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget, Advance Publications 2015).
Culturing tumor cells to predict treatment outcome
Cancer is among the leading causes of death in Singapore today. More than a hundred types of cancers have been identified, each with distinct characteristics and treatment challenges. Cancer results when genetic anomalies cause a few healthy cells to divide uncontrollably into a mass of abnormal cells called a tumor. As the cancer develops, some cells gain the ability to escape from the tumor, invade the bloodstream and subsequently squeeze out from the blood vessels to attach themselves to other parts of the body. This process, known as ‘metastasis’, is associated with poor prognosis and high mortality rates
Role of CTCs in cancer prognosis
The cells that manage to slip away from the tumor and enter the bloodstream are termed circulating tumor cells (CTCs). CTCs can be found even in the early stages of cancer and techniques that detect CTCs from patient blood can help in understanding cancer progression as well as predict patient response to cancer treatment. However, most conventional methods used to isolate CTCs from whole blood have lacked efficiency, as CTCs comprise of many sub-populations and occur at low frequencies in blood. Furthermore, the procedures used to exclusively identify certain sub-populations of CTCs from whole blood, are often unable to isolate all of the CTCs.
The ability to capture and efficiently grow CTCs from a blood sample will provide researchers and clinicians valuable tools to investigate the best therapy options for the patient.
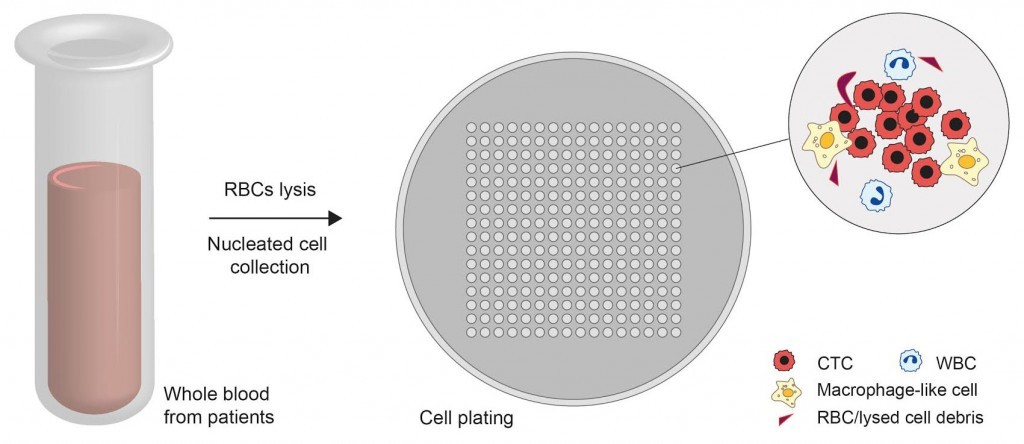
In an attempt to overcome these setbacks, MBI researchers developed a novel methodology for efficiently culturing CTCs from whole blood. In this method, CO2 lasers were used to engrave microwell patterns on a petri dish and whole blood samples from patients with early stage or metastatic breast cancer, which were treated beforehand to exclude the red blood cells, were deposited into the microwells. Most of the CTCs were found to be intact following this pre-treatment. The geometry of the microwells, the non-sticky nature of their surfaces, and the oxygen-deficient growth conditions provided an ideal environment for tumor cell growth. Other non-cancerous cells, such as white blood cells, gradually underwent cell death and were removed from the culture over time. Remarkably, the authors discovered the formation of cell clusters after two weeks of culture and the clusters primarily consisted of cells with characteristics of CTCs. Mechanistically, the cells increasingly resembled cancer cells with higher invasive characteristics, while their genetic profiles revealed cancer-causing mutations.
Tests were conducted on over 220 clinical samples from patients with both localized and metastatic breast cancers, and a success rate of over 60% in culturing CTCs was achieved. In patients undergoing cancer treatment, the authors observed that the formation of CTC clusters progressively declined with increase in treatment duration. This could be due to the clearing away of cancer cells from patient’s body, in response to treatment. In line with this, persistent cluster formation post-treatment suggests a poor response and poorer survival time.
The ability to capture and efficiently grow CTCs from a blood sample will provide researchers and clinicians valuable tools to investigate the best therapy options for the patient. This study describes a novel methodology to efficiently culture CTCs from patients with early stage or metastatic breast cancer. The combination of specially designed microwells and biologically suitable conditions provide an ideal microenvironment for CTC growth with many advantages over current methods. Along with other benefits such as shorter culture durations, low quantities of sample needed and a minimally invasive procedure, this technique is well-poised to be an ideal tool for assessing the status of cancer as well as for predicting the efficacy of specific treatment regimes.